From Petri Dishes to Digital PCR: The Evolving Battlefield of Pathogen Discovery
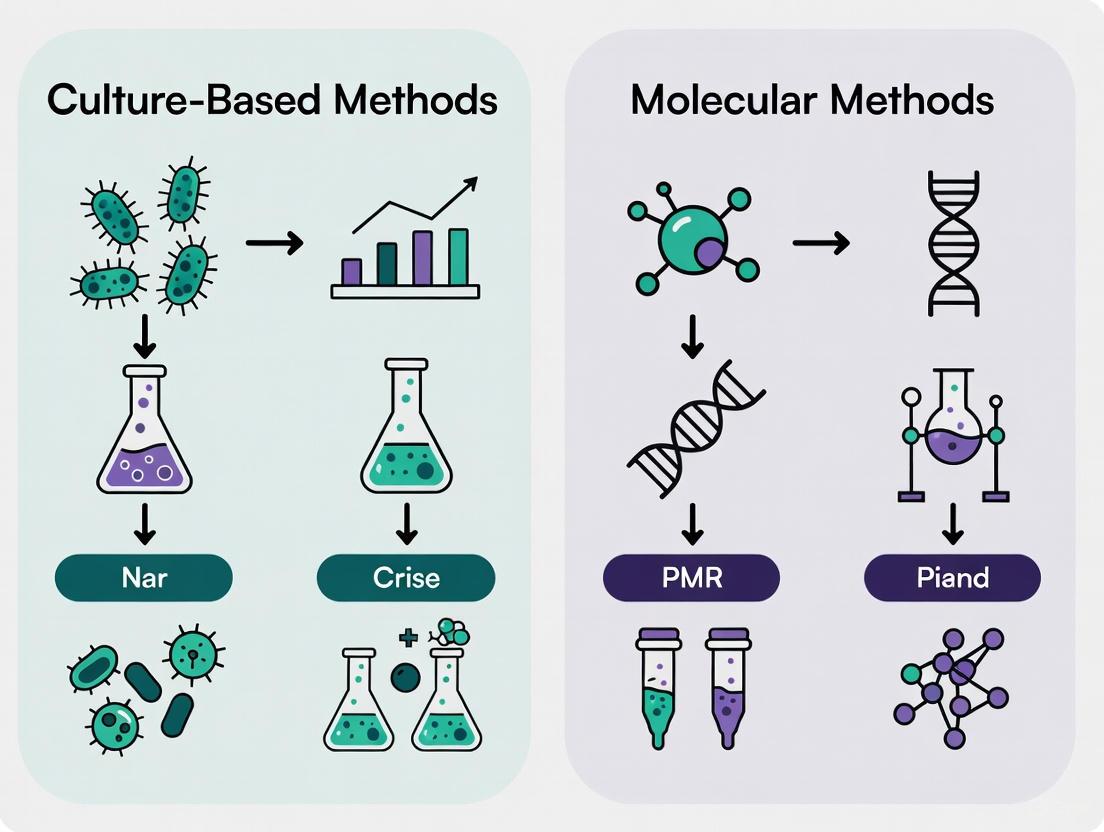
This article provides a comprehensive comparison between traditional culture-based methods and modern molecular techniques for pathogen discovery and diagnostics.
From Petri Dishes to Digital PCR: The Evolving Battlefield of Pathogen Discovery
Abstract
This article provides a comprehensive comparison between traditional culture-based methods and modern molecular techniques for pathogen discovery and diagnostics. Aimed at researchers, scientists, and drug development professionals, it explores the foundational principles, practical applications, and performance validation of these technologies. The content synthesizes recent evidence to illustrate a paradigm shift in clinical microbiology, highlighting how molecular methods like mNGS and ddPCR offer superior speed, sensitivity, and the ability to detect uncultivable pathogens, while also addressing their challenges and the enduring role of culture for antibiotic susceptibility testing.
The Microbiological Bedrock: Understanding Culture-Based Methods and Their Limitations
For over two centuries, traditional microbial culture has served as the foundational pillar of clinical microbiology, providing the critical link between microbial presence and infectious disease [1]. Despite the rapid ascent of molecular diagnostic techniques, culture methods remain the uncontested reference standard for a wide range of pathogens, offering unparalleled benefits for phenotypic characterization and antimicrobial susceptibility testing [1] [2]. The basic principle of microbial culture is to provide a suitable environment—including nutrients, temperature, and atmosphere—that supports the growth and multiplication of microorganisms from a clinical sample, allowing for their visualization, identification, and further analysis [1].
This guide objectively examines the position of traditional culture methodologies within the modern diagnostic and research landscape, directly comparing its performance against nucleic acid amplification techniques and next-generation sequencing. We present quantitative data on detection sensitivity, turnaround time, and clinical utility to provide researchers and drug development professionals with a clear framework for selecting appropriate pathogen discovery pathways.
Core Principles and Workflow of Traditional Culture
The workflow of traditional microbial culture is a systematic, multi-stage process designed to isolate, purify, and identify pathogens from complex clinical specimens. The foundational concepts of this methodology were established by pioneers like Pasteur and Koch, who developed the initial criteria for linking specific microbes to diseases [1]. The process leverages the ability to grow microorganisms in controlled laboratory conditions, either on inanimate media like agar plates for bacteria and fungi, or in animate systems like cell cultures for viruses and obligate intracellular pathogens [1].
Graphical representation of the multi-stage traditional culture workflow, from specimen processing to final reporting.
Key Methodological Steps
- Specimen Processing and Inoculation: Clinical samples are applied to solid or liquid culture media using tools like sterile loops or automated instruments (e.g., WASP, PREVI Isola) [3]. Automated systems demonstrate high agreement with manual methods (94.4-100%) and can provide better isolation quality, particularly for polymicrobial specimens [3].
- Incubation and Colony Isolation: Inoculated media are incubated at controlled temperatures for hours to days. Microbial growth appears as visible colonies on solid media or turbidity in liquid broths, which are then isolated for pure culture [1].
- Identification and Characterization: Colony morphology, Gram staining, and biochemical profiling form the basis for taxonomic identification. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) has revolutionized this step by enabling rapid, sensitive identification based on protein mass fingerprints [4].
- Antimicrobial Susceptibility Testing (AST): Pure isolates are subjected to phenotypic AST using disk diffusion or broth microdilution to determine minimum inhibitory concentrations (MICs), which are crucial for guiding effective antimicrobial therapy [5] [2].
Performance Comparison: Culture Versus Molecular Methods
The diagnostic landscape for infectious diseases has been transformed by molecular techniques that offer rapid turnaround times. The table below summarizes key performance metrics from recent comparative studies across different infection types and patient populations.
Table 1: Comparative performance of traditional culture versus molecular diagnostic methods for pathogen detection.
| Infection Type / Setting | Methodology | Detection Rate | Turnaround Time | Key Advantages | Major Limitations |
|---|---|---|---|---|---|
| Bacterial Meningitis [6] | Microbial Culture | 3% (10/400) | 48-72 hours | Enables AST; Gold standard | Low sensitivity |
| Real-time PCR | 10% (38/400) | 2-5 hours | High sensitivity; Rapid | No phenotypic AST | |
| Community-Acquired Pneumonia (HIV+) [5] | Sputum Culture | <25% | 48-72 hours | Guides targeted therapy | Low diagnostic yield |
| FilmArray Pneumonia Panel | 83.2% | ~2 hours | Detects viruses & resistance genes | Cannot differentiate colonization from infection | |
| Bloodstream Infection [7] | Blood Culture | 13.1% (13/99) | 5-7 days | Comprehensive live pathogen recovery | Lengthy process |
| mNGS | 65.7% (65/99) | 24-48 hours | Broad, culture-independent detection | High cost; Complex interpretation |
Analysis of Comparative Data
The data reveal a consistent pattern across clinical syndromes: molecular methods demonstrate superior detection sensitivity and dramatically shorter turnaround times compared to traditional culture. In bacterial meningitis, PCR detected nearly four times as many cases as culture (10% vs. 3%) [6]. Similarly, in HIV-positive patients with pneumonia, a multiplex PCR panel identified a potential bacterial etiology in over 83% of cases, compared to <25% for sputum culture [5].
However, culture maintains its critical role in antimicrobial stewardship. As noted in a recent evaluation, "Traditional methods were noted for their precision in determining minimum inhibitory concentrations (MICs), crucial for guiding effective antimicrobial therapy" [2]. This phenotypic information is particularly vital in an era of rising multidrug-resistant organisms, where molecular detection of resistance genes does not always correlate with phenotypic resistance patterns.
Detailed Experimental Protocols in Comparative Studies
Objective: To compare pathogen detection consistency between metagenomic next-generation sequencing (mNGS) and blood culture in patients with suspected bloodstream infection.
Methodology:
- Sample Collection: Blood samples for both tests were drawn on the same day from patients with fever (>38.5°C), chills, or antibiotic use >3 days.
- Blood Culture Protocol: Two sets of aerobic and anaerobic blood samples (5-10 mL each) were collected aseptically from different venipuncture sites. Cultures were incubated using the BACTECFX automated system for 5-7 days. Bacterial identification was performed using the VITEK MS system.
- mNGS Protocol: 6-8 mL of blood was collected and transported on dry ice. DNA was extracted using commercial kits (QIAamp DNA Micro Kit or QIAamp Microbiome DNA Kit). Libraries were prepared and sequenced on Illumina platforms (Nextseq 550 or NextSeq CN500). Bioinformatic analysis involved removing human sequences and aligning non-human reads to microbial databases.
- Positive Criteria: For mNGS, a microbe was reported positive if its coverage ranked in the top 10 of its kind AND was absent in negative controls, or if the RPMsample/RPMNTC ratio was >10.
Objective: To compare the diagnostic yield of the BioFire FilmArray Pneumonia Panel with conventional culture in hospitalized HIV-positive patients with community-acquired pneumonia.
Methodology:
- Sample Type: Sputum samples from hospitalized patients with HIV.
- Culture Method: Standard sputum culture protocols were followed with incubation for 24-48 hours. Isolates were identified using standard microbiological techniques.
- Molecular Method: The BioFire FilmArray Pneumonia Panel was used according to manufacturer's instructions. This multiplex PCR system detects genes of >20 bacterial pathogens and several resistance genes in approximately 2 hours.
- Outcome Measures: Primary outcomes were detection rates for bacterial pathogens and resistance markers. Secondary outcomes included identification of viral pathogens and mixed infections.
Essential Research Reagent Solutions and Materials
Successful implementation of traditional culture methods requires specific reagents and instruments optimized for different specimen types and microbial targets.
Table 2: Key research reagents and instruments for traditional microbial culture workflows.
| Item Category | Specific Examples | Function / Application | Key Characteristics |
|---|---|---|---|
| Culture Media | Agar plates, Broth media | Support microbial growth | Various formulations (e.g., blood agar, MacConkey) for selectivity |
| Automated Inoculation Systems | WASP (Copan), PREVI Isola (bioMérieux) | Standardized specimen plating | Reduces variability; Improves isolation quality [3] |
| Automated Blood Culture Systems | BACTECFX (Becton, Dickinson) | Continuous monitoring of liquid cultures | Detects microbial growth through gas production |
| Identification Systems | VITEK MS (BioMérieux) | MALDI-TOF mass spectrometry | Rapid identification based on protein fingerprints [4] |
| High-Throughput Screening | MilliDrop AzurEvo | Microbial phenotyping in droplets | Enables study of micro-communities; Monitors growth parameters [8] |
The Integrated Future of Pathogen Discovery
The reign of traditional microbial culture as the sole gold standard is evolving toward a collaborative diagnostic model where culture and molecular methods function synergistically. While molecular techniques excel in rapid detection and identification, culture remains indispensable for phenotypic characterization, antimicrobial susceptibility testing, and providing viable isolates for further research [1] [2].
Future advancements will likely focus on integrating these methodologies to leverage their complementary strengths. As noted in a recent perspective, "Revolutionary changes are on the way in clinical microbiology with the application of '-omic' techniques," including genomics, transcriptomics, proteomics, and metabolomics [1]. For researchers and drug development professionals, the strategic selection of methodology should be guided by the clinical or research question—opting for molecular speed when rapid identification is critical, while relying on culture's phenotypic capabilities when therapeutic guidance requires live isolates for comprehensive susceptibility testing.
The enduring reliance on cultivation-based methods in clinical microbiology presents a significant bottleneck for pathogen discovery and diagnostics. A substantial proportion of bacterial species, including fastidious organisms and those in a viable but non-culturable (VBNC) state, resist growth on standard laboratory media, leading to false-negative results and obscuring the true complexity of microbial infections. This guide objectively compares the performance of traditional culture techniques with modern molecular methods, leveraging experimental data to illustrate their respective capabilities in detecting and identifying pathogens. The analysis underscores a paradigm shift in clinical microbiology towards molecular techniques that circumvent the inherent constraints of culturability, thereby enhancing diagnostic accuracy and enabling more informed therapeutic interventions.
For over a century, the culture of microorganisms has been the cornerstone of clinical microbiology. However, the longstanding reliance on this method exists in stark contrast to the reality that the majority of the microbial world resists cultivation in the laboratory [9]. It is estimated that approximately 99% of environmental bacteria and around a third of oral bacteria remain uncultivated [10]. This "Great Plate Count anomaly," the discrepancy between microscopic cell counts and viable colony counts, highlights a fundamental limitation in our traditional approach to microbial diagnostics [10].
The challenges are twofold. First, fastidious organisms are those with complex and particular nutritional requirements, making them difficult to culture because it is difficult to accurately simulate their natural milieu in a culture medium [11]. Examples include Neisseria gonorrhoeae and Treponema pallidum [11]. Second, many bacteria, including major human pathogens, can enter a viable but non-culturable (VBNC) state under stress [12]. In this state, cells are metabolically active and viable, but cannot form colonies on conventional media routinely used for their detection, posing a significant threat to public health, food safety, and infectious disease management [12] [13]. This guide provides a comparative analysis of culture and molecular methods for pathogen discovery, framing them within the broader thesis that molecular techniques are essential for illuminating the vast, uncultivated microbial dark matter.
Defining the Challenge: Fastidious and VBNC Pathogens
Fastidious Organisms
A fastidious organism is defined by its complex nutritional requirements, meaning it will only grow when specific nutrients are present in its culture medium [11]. In practical terms, a fastidious microorganism is one that is "difficult to culture, by any method yet tried" [11]. The significance of fastidiousness is that a negative culture result could be a false negative; the organism's absence from culture does not prove its absence from the patient sample [11]. Many zoonotic and vector-transmitted bacteria are strongly adapted to the infected host, hindering pathogen cultivation and identification. These include obligate intracellular pathogens like Anaplasma spp., Bartonella spp., and Rickettsia spp., which require the intracellular compartment of eukaryotic host cells to replicate [14].
The Viable But Non-Culturable (VBNC) State
The VBNC state is a unique survival strategy adopted by many bacteria in response to adverse environmental conditions, such as temperature fluctuations, nutrient deprivation, and exposure to biocides [12] [13]. In this state, bacteria maintain metabolic activity and viability but are unable to proliferate on standard laboratory media, leading to an underestimation of their population density [12]. Cells in the VBNC state are distinct from dead cells; they preserve cellular integrity, retain genomic DNA, and exhibit metabolic activity [15]. A critical feature of many VBNC pathogens is that they can retain their virulence and, upon encountering favorable conditions, can resuscitate back to a culturable, infectious state [12]. This has been demonstrated for pathogens like Vibrio cholerae and Escherichia coli O157:H7, making them a concealed threat in water and food systems [12] [13].
Methodological Comparison: Culture vs. Molecular Diagnostics
Classical Culture-Based Diagnostics
Culture-based methods rely on growing microorganisms on or in nutrient-rich media (agar plates, liquid broths) under controlled conditions. For some fastidious organisms, this requires specialized media, specific atmospheric conditions (e.g., elevated CO2), or even eukaryotic host cells [14]. The process typically involves the following steps, which can take several days to weeks for slow-growing organisms:
- Sample Inoculation: The clinical specimen is plated onto solid and/or inoculated into liquid media.
- Incubation: Media are incubated under conditions designed to support the growth of suspected pathogens.
- Colony Isolation and Purification: Visible colonies are subcultured to obtain pure isolates.
- Identification: Isolates are identified based on morphological, biochemical, and sometimes antigenic properties.
- Antimicrobial Susceptibility Testing (AST): The pure isolate is tested against antibiotics to guide therapy.
Table 1: Limitations of Culture-Based Methods for Pathogen Detection
| Limitation | Impact on Diagnostic Outcome |
|---|---|
| Inability to culture VBNC cells | False-negative results; underestimation of pathogen load and risk [12] [13]. |
| Unmet growth requirements of fastidious organisms | False-negative results; failure to identify the causative agent [14] [11]. |
| Long turnaround time (days to weeks) | Delays in targeted therapeutic intervention, especially critical for slow-growing bacteria [14]. |
| Prior antibiotic exposure | Suppression of bacterial growth, leading to false-negative cultures [16]. |
| Difficulty in polymicrobial community analysis | Overgrowth by fast-growing species can mask the presence of slow-growing or fastidious pathogens [17]. |
Molecular (Culture-Independent) Diagnostics
Molecular methods detect microorganisms by identifying their genetic material (DNA or RNA), bypassing the need for cultivation. These techniques have emerged as powerful alternatives that overcome the constraints of culturability.
- Broad-Range PCR and 16S rRNA Gene Sequencing: This method uses polymerase chain reaction (PCR) with primers that target conserved regions of the bacterial 16S ribosomal RNA gene, amplifying the variable regions in between. The amplified product is then sequenced and compared to large databases for identification [9] [17]. This is particularly useful for identifying bacteria that are difficult to characterize biochemically.
- Metagenomic Next-Generation Sequencing (mNGS): This is a comprehensive approach that involves sequencing all the nucleic acids in a sample. After bioinformatic analysis to filter out human sequences, the remaining sequences are aligned against microbial databases to identify pathogens, including bacteria, viruses, fungi, and parasites [7]. mNGS does not require prior knowledge of the potential pathogen.
- Quantitative PCR (qPCR): This method uses PCR to detect and simultaneously quantify a specific pathogen by targeting a unique gene sequence. It is rapid and highly sensitive [16] [13].
Table 2: Key Advantages of Molecular Methods for Pathogen Detection
| Advantage | Diagnostic Impact |
|---|---|
| Does not require viability or culturability | Detects VBNC cells, dead cells, and fastidious organisms [12] [17]. |
| High sensitivity and specificity | Can detect low-abundance pathogens and provides precise identification [17] [7]. |
| Rapid turnaround time (hours to a few days) | Enables faster clinical decision-making and antibiotic stewardship [16] [7]. |
| Comprehensive profiling of polymicrobial infections | Reveals the full complexity of microbial communities in chronic wounds or biofilms [17]. |
| Detection of non-bacterial pathogens | A single test (e.g., mNGS) can identify viruses, fungi, and parasites from one sample [7]. |
Comparative Experimental Data: A Quantitative Analysis
Multiple studies have directly compared the output of culture and molecular methods, providing quantitative evidence of the limitations of traditional culturing.
Detection in Chronic Wounds
A retrospective study of 168 chronic wound samples compared aerobic bacterial culture with 16S rDNA sequencing [17]. The results demonstrated a dramatic increase in microbial diversity detected by molecular methods.
Table 3: Culture vs. Molecular Identification in Chronic Wounds [17]
| Method | Number of Bacterial Taxa Identified | Key Findings |
|---|---|---|
| Aerobic Culture | 17 | The majority of bacteria found by culture were also identified by molecular testing. |
| 16S rDNA Sequencing | 338 | The majority of bacteria (over 300 taxa) identified molecularly were not recovered by culture. |
The study concluded that molecular testing revealed an order of magnitude greater number of bacterial species than culture, fundamentally changing the understanding of the microbiota in chronic wound infections [17].
Detection in Bloodstream Infections
A 2023 study of 99 patients with suspected bloodstream infection compared pathogen detection using mNGS and blood culture [7]. The findings further underscore the sensitivity gap between the two methods.
Table 4: mNGS vs. Blood Culture in Suspected Bloodstream Infections [7]
| Method | Positive Detection Rate | Concordance for Bacteria/Fungi |
|---|---|---|
| Blood Culture | 13.13% (13/99 patients) | 12.00% |
| Blood mNGS | 65.66% (65/99 patients) |
The detection rate for pathogenic microorganisms by mNGS was significantly higher than that by blood culture (P < 0.001). Notably, mNGS identified viruses in 22 patients, a class of pathogen that blood culture cannot detect. The very low concordance rate highlights that the two methods often provide different diagnostic information [7].
Detailed Experimental Protocols
To ensure reproducibility and provide a clear technical reference, this section outlines the core methodologies cited in the comparative data.
This protocol is used for bacterial identification and community profiling in complex samples like chronic wound tissue.
DNA Extraction:
- Tissue samples are centrifuged and suspended in RLT buffer (Qiagen) with β-mercaptoethanol.
- Mechanical lysis is performed using a TissueLyser (Qiagen) with steel and glass beads.
- DNA is purified from the supernatant using the QIAamp DNA Mini Kit (Qiagen) following the manufacturer's tissue protocol.
- DNA is eluted in water and diluted to a standard concentration (e.g., 20 ng/μL).
16S rRNA Gene Amplification:
- Amplify the ~500 bp region of the 16S rRNA gene using modified universal bacterial primers (28F and 519R).
- Primer sets are modified with linker sequences and sample-specific barcodes to enable multiplexed sequencing on platforms like the FLX-Titanium (Roche).
- PCR is performed using a hot-start master mix (e.g., HotStarTaq Plus Master Mix Kit, QIAGEN) with 35 amplification cycles.
Sequencing and Data Analysis:
- Amplified products are pooled and subjected to pyrosequencing.
- Post-sequencing, raw data is processed to remove low-quality reads, adapter sequences, and primers.
- Filtered sequences are clustered into Operational Taxonomic Units (OTUs) based on sequence similarity.
- OTUs are classified by comparison to a curated 16S rRNA database (e.g., Greengenes, SILVA) to assign taxonomic identities.
This protocol is used for the untargeted detection of pathogens in blood samples.
Sample Collection and DNA Extraction:
- Collect 6-8 mL of blood in EDTA tubes.
- Extract cell-free DNA from plasma using a commercial kit (e.g., QIAamp DNA Micro Kit, QIAGEN).
- For comprehensive analysis, extract total DNA from blood samples using a dedicated microbiome DNA kit (e.g., QIAamp Microbiome DNA Kit).
Library Preparation and Sequencing:
- Construct DNA libraries using a kit designed for low-input or ultra-low input samples (e.g., QIAseq Ultralow Input Library Kit or Nextera XT DNA Library Prep Kit).
- Assess library quality using an instrument like the Agilent 2100 Bioanalyzer.
- Sequence the libraries on a high-throughput platform (e.g., Illumina Nextseq 550 or CN500).
Bioinformatic Analysis:
- Remove low-quality reads, adapter contamination, and duplicated reads.
- Align reads to the human reference genome (hg38 or hg19) using Burrows-Wheeler Aligner (BWA) and subtract them from subsequent analysis.
- The remaining non-host reads are aligned to comprehensive microbial genome databases (e.g., from NCBI).
- Establish positive detection criteria based on statistical thresholds, such as the ratio of reads per million (RPM) in the sample versus a negative control (RPMsample/RPMNTC > 10).
Visualization of Method Workflows
The following diagram illustrates the fundamental procedural differences between culture-based and molecular diagnostic pathways, highlighting the key reasons for their divergent outcomes.
Diagram 1: A comparative workflow of culture-based versus molecular pathogen detection pathways. The culture pathway is susceptible to several constraints that lead to false-negative results, whereas the molecular pathway bypasses the need for microbial growth.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table details key reagents and kits used in the molecular methods discussed, providing a resource for experimental design.
Table 5: Key Research Reagents for Molecular Pathogen Detection
| Reagent / Kit | Function in Experimental Protocol | Example Use Case |
|---|---|---|
| QIAamp DNA Mini Kit / Micro Kit | Extraction of high-quality genomic DNA from various sample types, including tissues and body fluids. | DNA extraction from chronic wound tissue [17] and plasma [7]. |
| QIAseq Ultralow Input Library Kit | Preparation of sequencing libraries from very low quantities of input DNA, critical for samples with low microbial biomass. | mNGS library prep from blood samples [7]. |
| Nextera XT DNA Library Prep Kit | Rapid preparation of sequenced-ready libraries from genomic DNA using a tagmentation-based approach. | mNGS library preparation [7]. |
| HotStarTaq Plus Master Mix Kit | A pre-mixed solution for PCR, containing a hot-start DNA polymerase for high specificity and yield in amplification. | Amplification of the 16S rRNA gene from wound samples [17]. |
| Burrows-Wheeler Aligner (BWA) | A bioinformatics software tool for aligning sequencing reads to large reference genomes, such as the human genome. | Filtering out human host sequences in mNGS data analysis [7]. |
| Microbial Genome Databases (NCBI) | Curated public databases of microbial reference sequences used for taxonomic classification of sequencing reads. | Identification of pathogens from non-host mNGS or 16S sequencing reads [17] [7]. |
| 2,4-Dimethylphenyl 2-ethoxybenzoate | 2,4-Dimethylphenyl 2-Ethoxybenzoate | Research Compound | High-purity 2,4-Dimethylphenyl 2-Ethoxybenzoate for research applications. This product is for Research Use Only (RUO) and is not intended for personal use. |
| 1-(3,4,5-Triethoxybenzoyl)pyrrolidine | 1-(3,4,5-Triethoxybenzoyl)pyrrolidine|High-Quality|RUO | 1-(3,4,5-Triethoxybenzoyl)pyrrolidine is a high-purity chemical building block for research. For Research Use Only. Not for human or veterinary use. |
The comparative data and experimental protocols presented in this guide objectively demonstrate the inherent constraints of culture-based methods, primarily their inability to detect VBNC cells and fastidious organisms. Molecular techniques like 16S rDNA sequencing and mNGS provide a more sensitive, comprehensive, and faster approach to pathogen discovery. They have fundamentally expanded our understanding of infectious diseases, revealing complex polymicrobial communities where culture once suggested simplicity or sterility. While culture remains important for obtaining isolates for antimicrobial susceptibility testing, the future of pathogen discovery and diagnostics is inextricably linked to the continued development and integration of these powerful molecular tools into clinical and research practice.
The Impact of Empirical Antibiotics on Culture Sensitivity and Diagnostic Delays
The initiation of empirical antibiotic therapy is a cornerstone of managing severe bacterial infections, particularly in critical care settings where delays in treatment are associated with significantly increased mortality [18] [19]. This life-saving intervention, however, presents a fundamental diagnostic dilemma: the very antibiotics administered to combat infections simultaneously compromise the sensitivity of gold-standard culture-based diagnostics and contribute substantially to treatment delays. This conflict creates a negative feedback loop that perpetuates broad-spectrum antibiotic use and complicates antimicrobial stewardship efforts.
Empirical therapy refers to the administration of antibiotics before a specific pathogen is identified, guided by clinical presentation and local resistance patterns [19]. While this approach is often necessary, it exerts selective pressure on microorganisms and can induce physiological changes that render them undetectable by conventional culture methods. The resulting diagnostic uncertainty frequently leads to prolonged empirical treatment, increased healthcare costs, and the acceleration of antimicrobial resistance (AMR) [18] [20]. This review examines the impact of empirical antibiotics on diagnostic efficacy, compares traditional and novel pathogen discovery methodologies, and explores how technological innovations are reshaping the diagnostic landscape to overcome these challenges.
The Diagnostic Challenge of Empirical Antibiotic Therapy
Clinical Rationale and Consequences of Empirical Treatment
The Surviving Sepsis Campaign guidelines recommend initiating empiric antibiotic therapy within one hour for patients with a high likelihood of sepsis, reflecting the time-sensitive nature of severe infection management [19]. International cohort studies demonstrate that approximately 75% of intensive care unit (ICU) patients with hospital-associated bloodstream infections receive empiric antibiotic therapy, with combination therapy employed in roughly half of these cases [19]. The decision to use empiric combination therapy is influenced by several patient-specific and institutional factors, including:
- Immune deficiency (OR 1.35)
- High SOFA scores >11 (OR 1.77)
- Uncommon infection sources (OR 1.63)
- Local resistance patterns, particularly high rates of carbapenemase-producing Enterobacteriaceae (OR 2.46) [19]
While often clinically necessary, this approach creates significant diagnostic challenges. A study by Vincent et al. revealed that more than 70% of ICU patients receive antibiotics, yet only about half of these treatments target documented infections [18]. This discrepancy highlights the substantial proportion of unnecessary antimicrobial exposure that occurs due to diagnostic uncertainty, contributing directly to the AMR crisis.
Impact of Antibiotics on Culture-Based Diagnostics
Culture-based methods remain the reference standard for pathogen identification but possess critical limitations when patients have received prior antibiotic therapy. Table 1 summarizes the primary mechanisms through which empirical antibiotics reduce culture sensitivity and cause diagnostic delays.
Table 1: Impact Mechanisms of Empirical Antibiotics on Culture-Based Diagnostics
| Impact Mechanism | Effect on Culture Sensitivity | Consequence for Diagnostic Delays |
|---|---|---|
| Reduced Microbial Viability | Induction of viable but non-culturable (VBNC) states; direct killing of susceptible organisms [18] [21] | False-negative results requiring repeat sampling; extended incubation periods |
| Delayed Time-to-Positivity | Sub-lethal antibiotic effects slow bacterial metabolism and replication [22] | Prolonged time to obtain actionable results (typically 48-72 hours) [18] |
| Altered Phenotypic Expression | Antibiotic pressure selects for resistant subpopulations not representative of original infection [20] | Inaccurate antimicrobial susceptibility profiles leading to inappropriate therapy |
| Masked Polymicrobial Infections | Suppression of susceptible species while resistant organisms proliferate [23] | Incomplete pathogen identification resulting in inadequate antimicrobial coverage |
The constraints of conventional techniques are particularly problematic in critical care settings, where the diagnostic window is narrow, and clinical deterioration can be rapid. Culture-based diagnostics typically require 48-72 hours or longer to yield actionable results, during which patients often receive prolonged, unnecessary broad-spectrum therapy [18]. This delay is especially consequential for infections caused by resistant organisms, where inadequate or delayed therapy is independently associated with higher mortality [18].
Comparative Analysis of Pathogen Detection Methods
Established Culture-Based Methodologies
Standard Protocols and Workflows
Conventional culture-based diagnosis typically begins with specimen inoculation onto selective and enriched media, followed by incubation for 24-48 hours to isolate pathogenic bacteria [20]. Automated biochemical testing systems then facilitate microbial identification, complemented by antimicrobial susceptibility testing (AST) using disk diffusion, broth microdilution, or gradient diffusion methods to guide therapeutic decisions [20]. The complete workflow from sample collection to AST results typically requires 48-72 hours, creating a critical therapeutic decision-making gap during the initial period of infection management.
The EUROBACT-2 international cohort study, which included data from 2406 adult patients across 328 ICUs in 52 countries, exemplifies the real-world application of these methods for diagnosing hospital-associated bloodstream infections [19]. In this context, blood culture serves as the fundamental diagnostic trigger, yet its utility is maximized only when followed by rapid and accurate identification and AST processes.
Key Research Reagent Solutions
Table 2: Essential Research Reagents for Culture-Based Diagnostics
| Reagent/Material | Function in Experimental Protocol | Specific Application Examples |
|---|---|---|
| Enriched & Selective Media | Isolation of pathogenic bacteria from clinical specimens by providing nutrients and inhibiting non-target organisms [20] | Blood agar, MacConkey agar, Chromogenic media for specific pathogen selection |
| Automated Biochemical Test Panels | Microbial identification based on metabolic capabilities and enzymatic activities [20] | API strips, VITEK systems, MALDI-TOF target plates |
| Antimicrobial Disks & Gradient Strips | Determination of antimicrobial susceptibility profiles through diffusion-based methods [20] [22] | Kirby-Bauer disks, Etest strips for MIC determination |
| Broth Microdilution Panels | Quantitative assessment of minimum inhibitory concentrations (MICs) in liquid culture [22] | CLSI-compliant panels for AST confirmation |
Modern Molecular Diagnostic Platforms
Technical Principles and Workflows
Molecular diagnostics have emerged as powerful alternatives to culture-based methods, particularly in the context of pre-exposure to empirical antibiotics. These platforms detect microbial nucleic acids or antigens rather than relying on viable organisms, thereby bypassing many limitations associated with antimicrobial pretreatment [18] [20].
Syndromic molecular panels represent one of the most significant advances, with platforms like the BioFire Blood Culture Identification (BCID) panel enabling simultaneous detection of multiple pathogens and resistance genes directly from positive blood cultures within hours [18]. Isothermal amplification techniques, such as loop-mediated amplification (LAMP), provide a cost-effective alternative to PCR-based methods, requiring less specialized equipment while maintaining high sensitivity [20].
Metagenomic sequencing represents the most comprehensive approach, allowing for culture-independent pathogen detection and resistance gene prediction. A recent study demonstrated a direct-from-blood-culture metagenomic sequencing method using Oxford Nanopore technology that achieved 97% sensitivity and 94% specificity for species identification, delivering results in just 3.5 hours—approximately one-third the time required by routine methods [23].
Key Research Reagent Solutions
Table 3: Essential Research Reagents for Molecular Diagnostics
| Reagent/Material | Function in Experimental Protocol | Specific Application Examples |
|---|---|---|
| Nucleic Acid Extraction Kits | Isolation and purification of pathogen DNA/RNA from clinical specimens while removing inhibitors [23] | Commercial kits for blood culture DNA extraction, automated extraction systems |
| Amplification Master Mixes | Provide optimized enzymes, buffers, and nucleotides for target-specific nucleic acid amplification [20] | PCR/LAMP reagents with integrated fluorescence detection |
| Hybridization Probes & Primers | Sequence-specific recognition of target pathogens or resistance genes through complementary binding [20] | TaqMan probes, molecular beacons, FISH probes |
| Sequence-Specific Reagents | Library preparation and barcoding for high-throughput sequencing applications [23] | Nanopore sequencing kits, Illumina library prep reagents |
The following workflow diagram illustrates the parallel pathways and critical decision points in traditional culture-based versus modern molecular diagnostic approaches:
Diagram 1: Comparative diagnostic workflows showing impact of empirical antibiotics.
Experimental Data and Performance Comparison
Quantitative Method Comparison
The performance disparities between culture-based and molecular diagnostic methods become particularly evident when analyzing key metrics across multiple studies. Table 4 presents a comparative analysis of these methodologies based on recent clinical evaluations.
Table 4: Performance Comparison of Diagnostic Methods for Pathogen Detection
| Methodology | Typical Turnaround Time | Sensitivity After Antibiotic Exposure | Key Advantages | Major Limitations |
|---|---|---|---|---|
| Culture & AST | 48-72 hours [18] | Significantly reduced due to VBNC state induction [18] [21] | Gold standard; provides live isolates for further testing; quantitative results [20] | Long turnaround time; low sensitivity post-antibiotics; unable to detect uncultivable organisms [18] |
| Rapid Molecular Panels | 1.5-6 hours [18] | Minimal impact (detects nucleic acids) [18] [20] | Rapid results; high sensitivity/specificity; detects resistance genes directly [18] | Limited target spectrum; higher cost; may miss novel resistance mechanisms [20] |
| Metagenomic Sequencing | 3.5-24 hours [23] | Minimal impact (detects nucleic acids) [23] | Comprehensive pathogen detection; discovers novel organisms; predicts AMR from genetic markers [23] | High cost; complex data analysis; requires specialized expertise [23] |
| AI-Powered Diagnostics | Minutes to hours [24] | Minimal impact (analysis of direct signatures) [24] | Ultra-rapid analysis; pattern recognition; predictive analytics for resistance [24] | Early development stage; validation limited; infrastructure requirements [24] |
Impact on Clinical Outcomes
The diagnostic delays inherent to culture-based methods have demonstrable clinical consequences. Studies indicate that inadequate or delayed therapy in the presence of resistant pathogens is independently associated with higher mortality, particularly in bloodstream infections and ventilator-associated pneumonias [18]. Infections caused by resistant organisms are associated with increased morbidity, length of ICU stay, mechanical ventilation duration, and healthcare costs [18].
Conversely, rapid diagnostic technologies have demonstrated significant benefits. Metagenomic sequencing applied directly to blood cultures detected 18 additional infections—13 polymicrobial and 5 previously unidentifiable—compared to standard methods, while delivering findings 20 hours faster for antimicrobial resistance prediction [23]. For key pathogens like Staphylococcus aureus and Escherichia coli, this approach achieved AMR prediction sensitivities of 100% and 91%, and specificities of 99% and 94%, respectively [23].
Emerging Technologies and Future Directions
Novel Diagnostic Platforms
Artificial intelligence (AI) is poised to revolutionize bacterial diagnostics and antimicrobial susceptibility testing. AI-powered platforms can automate the analysis of complex datasets from various diagnostic modalities, including microscopy, mass spectrometry, and Raman spectroscopy, enabling faster and more accurate bacterial identification [24]. Machine learning algorithms, such as convolutional neural networks (CNNs) and support vector machines (SVMs), have been successfully applied to tasks ranging from colony counting on agar plates to analyzing surface-enhanced Raman spectroscopy (SERS) data for antibiotic resistance detection [24].
Biosensor technologies represent another promising frontier, offering the potential for rapid, point-of-care pathogen detection. These systems transduce microbial signatures into measurable signals, with electrochemical, optical, and mass-sensitive biosensors showing particular promise for clinical application [24]. When integrated with AI for data interpretation, these platforms could enable real-time pathogen identification and resistance profiling at the bedside, fundamentally transforming infection management.
Advanced Therapeutic Discovery
The challenges of antimicrobial resistance have spurred innovation in therapeutic discovery, with deep learning approaches now enabling the mining of proteomic data for novel antibiotic candidates. The APEX (antibiotic peptide de-extinction) platform utilizes multitask deep learning to predict antimicrobial activity from peptide sequences, successfully identifying 37,176 sequences with predicted broad-spectrum activity from extinct organism proteomes [25]. Experimental validation confirmed the activity of 69 synthesized peptides, with several showing efficacy in mouse infection models [25].
These computational approaches are particularly valuable given the unique challenges of antibiotic discovery, including the restrictive penetration barrier that makes conventional screening of synthetic compound libraries largely impractical [26]. By leveraging AI to explore previously untapped sequence spaces, researchers can accelerate the identification of novel therapeutic candidates to address the growing threat of antimicrobial resistance.
The tension between empirical antibiotic therapy and diagnostic sensitivity represents a fundamental challenge in modern infectious disease management. While culture-based methods remain essential for antimicrobial susceptibility testing, their limitations in the context of pretreatment with empirical antibiotics are increasingly apparent. Molecular diagnostics, including syndromic panels and metagenomic sequencing, offer compelling advantages in speed and sensitivity, particularly for patients who have already received antimicrobial therapy.
The integration of these advanced diagnostic platforms into antimicrobial stewardship programs is crucial for optimizing therapy, improving patient outcomes, and combating the global rise of antimicrobial resistance. Future developments in AI-powered diagnostics and biosensor technologies promise to further bridge the gap between therapeutic intervention and pathogen identification, potentially enabling a new paradigm of precision infectious disease management. As these technologies mature and become more accessible, they offer the potential to resolve the critical conflict between immediate therapeutic needs and long-term diagnostic efficacy.
The cornerstone of microbiology, culture-based methods, has long provided the foundation for pathogen discovery. However, a paradigm shift is underway. It is now established that a vast majority of microbial life, often referred to as "microbial dark matter," resists cultivation under standard laboratory conditions [27] [28]. This review objectively compares the performance of traditional culture-based techniques with modern molecular methods for pathogen discovery. We synthesize experimental data demonstrating how molecular techniques reveal significantly greater microbial diversity, while culture-based methods remain vital for obtaining isolates for phenotypic studies. By integrating recent findings from clinical and environmental studies, this guide provides a framework for researchers to select appropriate methodologies, highlighting that an integrated approach offers the most comprehensive assessment of microbial communities.
The "great plate count anomaly" – the observation that typically less than 2% of environmental microorganisms form colonies on agar plates – highlights a fundamental limitation in traditional microbiology [29]. This is not merely a technical hurdle; it represents a critical gap in our understanding of life on Earth, particularly in the human microbiome where an estimated 40-50% of species lack a reference genome [28]. In clinical settings, this bottleneck has direct consequences for patient care, as standard diagnostic cultures may miss fastidious, slow-growing, or non-cultivatable pathogens, potentially leading to inadequate antimicrobial treatments, especially in polymicrobial infections where mortality risk increases by 2-3 folds compared to monomicrobial infections [30]. The emergence of sophisticated molecular techniques is now dismantling this barrier, enabling researchers to explore the immense diversity of uncultured microorganisms and their potential roles in health, disease, and biotechnology.
Methodological Comparison: Culture-Based vs. Molecular Approaches
Fundamental Principles and Workflows
The core distinction between these methodologies lies in their basic approach: culture-based methods rely on microbial growth and replication in artificial media, while molecular techniques detect microbial DNA or RNA directly from samples.
Culture-Based Methods require the selection of appropriate growth media and incubation conditions (e.g., aerobic, anaerobic, specific temperatures) to support the replication of microorganisms. The process involves sample inoculation, incubation, and subsequent analysis of grown colonies through morphological examination and biochemical tests. Advanced cultivation techniques for anaerobes, such as the roll-tube method and the use of anaerobic chambers with atmospheres of 95% nitrogen and 5% hydrogen, have improved recovery but remain technically challenging [29].
Molecular Methods bypass the need for cultivation by directly analyzing genetic material. Key approaches include:
- PCR-Based Techniques (Traditional PCR, qPCR, ddPCR): Amplify and detect specific target DNA sequences.
- Sequencing-Based Techniques: Metagenomic Next-Generation Sequencing (mNGS) sequences all DNA in a sample, enabling broad pathogen detection without prior knowledge of organisms present [7].
- Hybrid Methods: Culture-enriched metagenomic sequencing (CEMS) involves culturing samples on multiple media, pooling all grown colonies, and then performing metagenomic sequencing, thus combining growth enrichment with comprehensive genetic analysis [31].
The workflow differences are substantial, as illustrated below:
Performance Metrics and Experimental Data
Multiple studies have quantitatively compared the detection capabilities of culture-based and molecular methods across various sample types. The following table synthesizes key comparative findings:
Table 1: Comparative Performance of Culture-Based vs. Molecular Methods in Pathogen Detection
| Study Context | Culture-Based Detection Rate | Molecular Method Detection Rate | Key Findings | Reference |
|---|---|---|---|---|
| Suspected Bloodstream Infection (n=99) | 13.13% (13/99 patients) | 65.66% (65/99 patients); mNGS | mNGS detected viruses in 22 patients; concordance for bacteria/fungi only 12% | [7] |
| Chronic Diabetic Foot Wounds (n=26) | 50% (13/26 wounds) | Significantly greater bacterial diversity (P<0.05); DGGE | Molecular methods detected Staphylococcus sp. in 11/13 putatively uninfected wounds | [32] |
| Necrotizing Soft Tissue Infections (n=20) | 70% of samples | 90% of samples; Multiple molecular methods | Molecular methods frequently detected additional microorganisms | [16] |
| Human Gut Microbiome (n=1) | 36.5% of species (CEMS) | 45.5% of species (CIMS); Metagenomic sequencing | Only 18% species overlap between methods; both approaches essential | [31] |
| Global Human Gut Microbiome | Reference genomes for ~50% of phylogenetic diversity | 60,664 MAGs identified 2,058 novel species; 50% increase in diversity | Newly identified species accounted for 28% of abundance per individual | [28] |
The data consistently demonstrate molecular methods' superior sensitivity for detecting microbial diversity, though culture remains indispensable for isolating viable organisms.
Technical Protocols for Microbial Diversity Assessment
Culture-Enriched Metagenomic Sequencing (CEMS)
Sample Preparation and Cultivation:
- Fresh fecal sample (0.5 g) is homogenized in 4.5 g distilled water and serially diluted (10â»Â³ to 10â»â·) in 0.85% NaCl solution [31].
- Aliquot 200 µL of each dilution onto 12 different commercial or modified media types, including nutrient-rich media (LGAM, PYG), selective media (MAR with high salt), and oligotrophic media (1/10GAM) [31].
- Incubate duplicate sets anaerobically (95% N₂, 5% H₂) and aerobically at 37°C for 5-7 days [31].
Metagenomic Analysis:
- Harvest all colonies from each medium using a cell scraper with 0.85% NaCl solution [31].
- Extract DNA using the QIAamp Fast DNA Stool Mini Kit following manufacturer's instructions [31].
- Perform shotgun metagenomic sequencing using Illumina HiSeq 2500, generating 100 bp paired-end reads [31].
- Process data through HUMANN2 pipeline using MetaPhlAn2 for microbial composition profiling [31].
PCR-Denaturing Gradient Gel Electrophoresis (DGGE)
DNA Extraction and Amplification:
- Extract DNA from clinical samples using a DNeasy blood and tissue kit [32].
- Amplify the V2-V3 region of the 16S rRNA gene using eubacterium-specific primers HDA1 (with GC clamp) and HDA2 [32].
- Thermal amplification program: 94°C for 4 minutes; 30 cycles of 94°C for 30s, 56°C for 30s, 68°C for 60s; final elongation at 68°C for 7 minutes [32].
DGGE Analysis:
- Perform polyacrylamide electrophoresis with 30% and 60% denaturing concentrations using the DCode Universal Mutation Detection System [32].
- Analyze banding patterns to assess microbial diversity; excise and sequence distinctive bands for taxonomic identification [32].
Metagenomic Next-Generation Sequencing (mNGS) for Bloodstream Infections
Sample Processing:
- Collect 6-8 mL of blood in sterile containers [7].
- Extract DNA using QIAamp DNA Micro Kit or QIAamp Microbiome DNA Kit [7].
- Construct libraries using QIAseq Ultralow Input Library Kit or Nextera XT DNA Library Prep Kit [7].
Sequencing and Analysis:
- Sequence on Illumina Nextseq platform (100-150 bp reads) [7].
- Remove adapter sequences, low-quality reads, and human host sequences by alignment to hg38/hg19 reference [7].
- Align remaining reads to microbial genome databases; positive detection criteria vary by microbe type but generally require RPMsample/RPMNTC ratio >10 [7].
Essential Research Reagent Solutions
Successful assessment of uncultured microbial diversity requires specific reagents and tools. The following table catalogizes key solutions and their applications:
Table 2: Essential Research Reagents for Microbial Diversity Studies
| Reagent/Tool Category | Specific Examples | Function/Application | Reference |
|---|---|---|---|
| DNA Extraction Kits | DNeasy Blood & Tissue Kit, QIAamp DNA Micro Kit, QIAamp Fast DNA Stool Mini Kit | High-quality DNA extraction from diverse sample types | [32] [31] [7] |
| PCR Reagents | HDA1/HDA2 primers, Red Taq DNA polymerase ready mix | Amplification of 16S rRNA gene regions for diversity analysis | [32] |
| Specialized Growth Media | LGAM, PYG, GAM, MRS-L, RG, 1/10GAM | Cultivation of diverse microbial communities under various nutritional conditions | [31] |
| Library Preparation Kits | QIAseq Ultralow Input Library Kit, Nextera XT DNA Library Prep Kit | Preparation of sequencing libraries from low-input DNA samples | [7] |
| Bioinformatic Tools | HUMANN2, MetaPhlAn2, DIAMOND, IGGsearch | Taxonomic profiling, functional analysis, and quantification of microbial abundance | [31] [28] |
| Anaerobic System Components | Anaerobic chamber (95% Nâ‚‚, 5% Hâ‚‚), roll tubes, pre-reduced media | Cultivation of oxygen-sensitive anaerobic microorganisms | [29] |
Integrated Analysis and Future Directions
The experimental data consistently demonstrate that molecular methods detect a substantially greater proportion of microbial diversity than culture-based methods alone. In suspected bloodstream infections, mNGS demonstrated a 5-fold higher detection rate compared to blood culture (65.66% vs. 13.13%) [7]. In gut microbiome studies, culture-enriched metagenomic sequencing (CEMS) and direct culture-independent metagenomic sequencing (CIMS) showed limited overlap (18% of species), indicating these methods access different components of the microbial community [31].
The clinical implications are significant. Molecular methods can identify pathogens in cases where cultures remain negative, particularly after antibiotic administration [16]. Furthermore, they reveal the polymicrobial nature of many infections, which is crucial since polymicrobial bloodstream infections increase mortality risk by 2-3 folds compared to monomicrobial infections [30].
However, culture-based methods provide irreplaceable benefits: they yield live isolates for antimicrobial susceptibility testing, functional characterization, and detailed physiological studies [29]. The future of pathogen discovery lies in integrated approaches that leverage the strengths of both methodologies. Culturing the uncultured requires innovative strategies such as growth-curve-guided cultivation, which uses real-time monitoring to identify optimal isolation points for slow-growing organisms before they are outcompeted [29].
As molecular technologies continue to advance—with improvements in long-read sequencing, single-cell genomics, and bioinformatic analysis—our ability to explore the vast uncultured microbial diversity will expand exponentially, opening new frontiers for drug discovery, microbiome research, and clinical diagnostics.
The Molecular Toolkit: PCR, Sequencing, and CRISPR in Modern Pathogen Detection
The shift from traditional culture methods to molecular techniques represents a fundamental evolution in pathogen discovery research. While culture-based methods have long been the gold standard, they are often time-consuming, limited in sensitivity, and unable to detect unculturable organisms. The advent of quantitative PCR (qPCR) introduced a new era of speed and specificity, yet this method still relied on relative quantification against standard curves, introducing potential variability. The emergence of digital PCR (dPCR) and its droplet-based counterpart ddPCR now enables absolute quantification of nucleic acids without standard curves, offering unprecedented precision for applications ranging from viral load monitoring to rare mutation detection [33] [34] [35]. This technological revolution provides researchers with powerful tools to address the limitations of both conventional culture methods and earlier molecular techniques, particularly for challenging samples with low pathogen concentrations or significant inhibitor content.
Fundamental Principles: How dPCR Achieves Absolute Quantification
The Partitioning Principle of Digital PCR
Digital PCR operates on a fundamentally different principle than qPCR. Rather than monitoring amplification in real-time through cycle threshold (Ct) values, dPCR partitions a single PCR reaction into thousands to millions of individual reactions [36] [34]. This partitioning occurs through various mechanisms including water-oil emulsion droplets (ddPCR), fixed micro-wells (nanoplate dPCR), or microfluidic chips (cdPCR) [36]. Each partition ideally contains zero or one (or a few) target DNA molecules. After end-point PCR amplification, each partition is analyzed as either positive (containing the target sequence) or negative (not containing the target) [34]. The ratio of positive to total partitions, analyzed using Poisson statistics, allows for the absolute quantification of the target nucleic acid in the original sample without the need for standard curves [33] [34].
Key Technological Variations in dPCR Platforms
The dPCR landscape encompasses several partitioning technologies, each with distinct advantages:
- Droplet Digital PCR (ddPCR): Uses immiscible fluids to generate tens of thousands of nanoliter-sized droplets that serve as individual reaction chambers [36] [33]. This approach typically generates the highest number of partitions but involves multiple instruments and transfer steps.
- Nanoplate-based dPCR: Employs microfluidic digital PCR plates with predefined wells (e.g., 8,500-26,000 partitions) [36]. This integrated system combines partitioning, thermocycling, and imaging in a single instrument, offering a streamlined workflow similar to qPCR.
- Chip-based dPCR (cdPCR): Utilizes microfluidic chips with thousands of nanoliter reaction chambers [36]. This approach offers fast partitioning but may involve more complex fluidics.
- Crystal Digital PCR: A hybrid approach combining cdPCR's 2D array format with droplet partitions, creating monolayer arrays of monodisperse droplets [36].
Table 1: Comparison of Major dPCR Partitioning Methods
| Partitioning Method | Number of Partitions | Volume of Partitions | Key Principles |
|---|---|---|---|
| Nanoplate | 8,500-26,000 | ~10 nL | Microfluidic digital PCR plate |
| Droplet generator | 10,000-1,000,000+ | 10-100 pL | Water-oil emulsion droplets |
| Microfluidic chambers | ~1,000,000 | ~10 nL | Pinning of oil interface to isolate chambers |
| Open arrays of microwells | ~100,000 | ~10 nL | Capillary action loading |
| CZL55 | CZL55, MF:C20H22N2O6, MW:386.4 g/mol | Chemical Reagent | Bench Chemicals |
| FM19G11 | FM19G11, CAS:329932-55-0, MF:C23H17N3O8, MW:463.4 g/mol | Chemical Reagent | Bench Chemicals |
Comparative Performance: dPCR vs. qPCR in Diagnostic Applications
Tuberculosis Diagnostics: Enhanced Detection of Paucibacillary Disease
A 2023 meta-analysis of 14 diagnostic accuracy studies compared ddPCR and qPCR for detecting pulmonary and extrapulmonary tuberculosis [35]. The analysis included 1,672 participants or biological samples and 975 tuberculosis events. While qPCR demonstrated higher sensitivity [0.66 (95% CI 0.60-0.71)] compared to ddPCR [0.56 (95% CI 0.53-0.58)], ddPCR showed superior discriminant capacity with a higher area under the ROC curve (0.97 for ddPCR vs. 0.94 for qPCR, p=0.002) [35]. This difference was particularly pronounced for extrapulmonary tuberculosis, where traditional methods often fail due to extremely low acid-fast bacilli concentrations [35]. The absolute quantification capability of ddPCR provides significant advantages for paucibacillary samples where accurate quantification at low target concentrations is critical for diagnosis and treatment monitoring.
Respiratory Virus Detection During the 2023-2024 Tripledemic
A 2025 study comparing dPCR and Real-Time RT-PCR for detecting influenza A, influenza B, RSV, and SARS-CoV-2 during the 2023-2024 tripledemic demonstrated dPCR's superior quantification accuracy, particularly for samples with high viral loads [37]. The study analyzed 123 respiratory samples stratified by cycle threshold (Ct) values into high (Ct≤25), medium (Ct25.1-30), and low (Ct>30) viral load categories. dPCR demonstrated superior accuracy for high viral loads of influenza A, influenza B, and SARS-CoV-2, and for medium loads of RSV [37]. The technology showed greater consistency and precision than Real-Time RT-PCR, especially in quantifying intermediate viral levels, highlighting its potential for precise viral load monitoring in co-infection scenarios [37].
Shiga Toxin-Producing E. coli Detection in Environmental Samples
Research on Shiga toxin-producing E. coli (STEC) detection in environmental samples demonstrated ddPCR's superior sensitivity for low-concentration targets [33]. While recombinase polymerase amplification (RPA) detected <10 CFU/mL and qPCR quantified from 10³ to 10ⷠCFU/mL, ddPCR showed quantification from 1 to 10ⴠCFU/mL with high reproducibility [33]. This study also highlighted ddPCR's greater tolerance to inhibitors present in complex environmental matrices like dairy lagoon effluent and high-rate algae pond effluent. Unlike qPCR, which is highly dependent on amplification efficiency and can be significantly affected by inhibitors, ddPCR's endpoint measurement and partitioning approach reduce the impact of substances that would normally inhibit PCR amplification [33].
Table 2: Comparative Performance of qPCR and dPCR Across Applications
| Application | qPCR Performance | dPCR/ddPCR Performance | Key Advantage of dPCR |
|---|---|---|---|
| Tuberculosis Diagnosis | Sensitivity: 0.66, Specificity: 0.98 [35] | Sensitivity: 0.56, Specificity: 0.97, AUC: 0.97 [35] | Superior discriminant capacity (AUC), especially for extrapulmonary TB |
| Respiratory Virus Detection | Variable quantification based on standard curves [37] | Superior accuracy for high viral loads, greater consistency [37] | Absolute quantification without standard curves, precision at intermediate viral loads |
| STEC Detection in Environmental Samples | Quantification from 10³ to 10ⷠCFU/mL [33] | Quantification from 1 to 10ⴠCFU/mL, high reproducibility [33] | Enhanced sensitivity for low-concentration targets, better inhibitor tolerance |
| Gene Expression Analysis | Relative to reference genes [38] | Absolute quantification without reference genes [38] | No need for stable reference genes, direct comparison across experiments |
Technical Comparison: dPCR vs. ddPCR Platforms and Workflows
Workflow Efficiency and Practical Implementation
The choice between dPCR and ddPCR platforms involves significant practical considerations for laboratory workflow:
- dPCR (Nanoplate Systems): Offers integrated, automated workflows with complete processing in 2-3 hours [36] [39]. Systems like the QIAcuity provide "sample-in, results-out" processing in a single instrument, significantly reducing hands-on time and contamination risk [36] [39]. This streamlined approach is particularly valuable for quality control environments and regulated laboratories.
- ddPCR (Droplet Systems): Typically involves multiple instruments (droplet generator, thermocycler, droplet reader) and requires 6-8 hours for complete processing [36] [39]. The workflow includes multiple pipetting and transfer steps, increasing the risk of cross-contamination and requiring trained personnel [36]. However, ddPCR can generate a higher number of partitions (up to millions), potentially increasing sensitivity for rare targets [36].
Multiplexing Capability and Data Quality
Modern dPCR platforms offer enhanced multiplexing capabilities compared to earlier ddPCR systems. Nanoplate-based dPCR systems can detect up to 5-12 targets in a single reaction, allowing simultaneous measurement of multiple biomarkers [36] [39]. While newer ddPCR models have improved multiplexing capabilities (up to 12 targets), they traditionally have been more limited in this regard [39]. Data quality can also be affected by technical issues specific to each platform. ddPCR may suffer from "rain" droplets (intermediate fluorescence signals) caused by damaged droplets, non-specific amplification, or irregular droplet size, complicating threshold setting [36]. Nanoplate dPCR eliminates variability associated with droplet size and coalescence, potentially offering more robust and reproducible results [36].
The Scientist's Toolkit: Essential Reagent Solutions for dPCR
Successful implementation of dPCR technologies requires specific reagent systems optimized for partition-based amplification:
Table 3: Essential Research Reagent Solutions for Digital PCR
| Reagent Type | Function | Application Examples |
|---|---|---|
| Primer-Probe Mixes | Target-specific amplification and detection | Commercial multiplex assays for respiratory viruses (Influenza A/B, RSV, SARS-CoV-2) [37] |
| dPCR Master Mixes | Optimized for partition-based amplification | Pre-mixed master mixes with digital PCR optimizers [36] |
| Partitioning Fluids | Create stable emulsion (ddPCR) or seal partitions | Immiscible oils for droplet stabilization [36] |
| Reference Assays | Quality control of sample processing | Internal positive controls for extraction and amplification [37] |
| Quantitative Standards | Assay validation and performance verification | Synthetic nucleic acids with known copy numbers [34] |
| Lixisenatide | Lixisenatide, CAS:320367-13-3, MF:C215H347N61O65S, MW:4858 g/mol | Chemical Reagent |
| Lipofermata | Lipofermata, MF:C15H10BrN3OS, MW:360.2 g/mol | Chemical Reagent |
The revolution from standard to digital PCR represents a significant advancement in quantification capabilities for pathogen discovery research. While qPCR remains suitable for many high-throughput screening applications, dPCR technologies provide superior precision for absolute quantification, particularly for low-abundance targets, complex matrices with inhibitors, and situations requiring exact copy number determination without reference standards [33] [35] [37]. The choice between dPCR platforms should be guided by specific application requirements: nanoplate-based systems offer streamlined workflows advantageous for quality control and regulated environments [36] [39], while droplet-based systems provide maximum partitioning density for detecting rare targets [36]. As these technologies continue to evolve with improved multiplexing capabilities, reduced costs, and enhanced automation, dPCR is poised to become an increasingly indispensable tool in the molecular pathology toolkit, bridging the gap between traditional molecular methods and the emerging era of precision pathogen quantification.
The field of pathogen discovery has undergone a revolutionary transformation with the advent of metagenomic next-generation sequencing (mNGS). This hypothesis-free approach represents a fundamental departure from both traditional culture-based methods and targeted molecular diagnostics, enabling comprehensive detection of pathogens without prior knowledge of the causative agent [40]. While conventional microbiological testing (CMT) has long served as the cornerstone of infectious disease diagnosis, its limitations—including prolonged turnaround times, low sensitivity for fastidious organisms, and the requirement for specific growth conditions—have driven the development of more agnostic detection methods [41]. The ongoing comparison between culture and molecular methods for pathogen discovery now centers on how mNGS complements and enhances traditional approaches, particularly for difficult-to-diagnose infections where conventional methods often fail to identify causative pathogens [42].
mNGS operates on a simple yet powerful principle: by sequencing all nucleic acids in a clinical sample and computationally subtracting human sequences, it can detect any pathogen—bacterial, viral, fungal, or parasitic—present in the sample [40]. This unbiased nature makes it particularly valuable for diagnosing unusual infections, detecting emerging pathogens, and identifying co-infections that might be missed by targeted approaches [43]. As the technology has evolved from research settings to clinical applications, understanding its performance characteristics relative to established methods has become essential for researchers, clinical microbiologists, and infectious disease specialists seeking to implement this powerful tool in diagnostic and research pipelines.
Performance Comparison: mNGS Versus Conventional Diagnostic Methods
Comprehensive Diagnostic Performance Metrics
Extensive clinical studies across diverse infection types and sample matrices have demonstrated that mNGS consistently outperforms conventional methods in sensitivity while maintaining high specificity, though its performance varies depending on the clinical context and pathogen type.
Table 1: Overall Diagnostic Performance of mNGS Across Various Infection Types
| Infection Type | Reference Standard | mNGS Sensitivity | mNGS Specificity | Conventional Method Sensitivity | Conventional Method Specificity |
|---|---|---|---|---|---|
| Central Nervous System Infections [44] | Clinical composite | 63.1% | 99.6% | 45.9% (CSF direct detection) | - |
| Spinal Infections [45] | Histopathology/IDSA criteria | 81% | 75% | 34% (Tissue Culture) | 93% |
| Pulmonary Infections [41] | Clinical composite | 78.89% (etiological diagnosis rate) | - | 20% (etiological diagnosis rate) | - |
| Bloodstream Infections [7] | Clinical composite | 65.66% (positivity rate) | - | 13.13% (positivity rate) | - |
The superior sensitivity of mNGS is particularly evident in challenging clinical scenarios. In a seven-year analysis of 4,828 cerebrospinal fluid (CSF) samples, mNGS demonstrated significantly higher sensitivity (63.1%) compared to indirect serologic testing (28.8%) and direct detection testing from both CSF (45.9%) and non-CSF samples (15.0%) [44]. Notably, 21.8% of infectious diagnoses would have been missed without mNGS testing, underscoring its unique value in detecting pathogens that evade conventional methods [44].
Pathogen-Specific Performance Variations
The diagnostic performance of mNGS varies considerably across pathogen types, reflecting differences in microbial burden, nucleic acid extraction efficiency, and background host DNA.
Table 2: Pathogen-Specific Detection by mNGS in CNS Infections (7-Year Study of 4,828 Samples) [44]
| Pathogen Category | Number Detected | Percentage of Total | Notable Pathogens Detected |
|---|---|---|---|
| DNA Viruses | 363 | 45.5% | Herpesviruses, Polyomaviruses |
| RNA Viruses | 211 | 26.4% | HIV, Arboviruses, Enteroviruses |
| Bacteria | 132 | 16.6% | Mycobacterium tuberculosis, Borrelia burgdorferi |
| Fungi | 68 | 8.5% | Coccidioides spp., Cryptococcus spp. |
| Parasites | 23 | 2.9% | Balamuthia mandrillaris |
For specific pathogens, mNGS shows exceptional capability. In tuberculosis detection, mNGS demonstrated 92.31% sensitivity compared to a composite reference standard, rivaling real-time PCR (90.38%) while maintaining perfect specificity (100%) [46]. The strong negative correlation between mNGS standardized microbial read numbers (SMRNs) and PCR cycle threshold values further validates its quantitative potential [46].
In immunocompromised populations, mNGS shows particular utility. For people living with HIV/AIDS with CNS disorders, mNGS achieved a positivity rate of 75% compared to 52.1% with conventional methods, with significantly improved detection of multiple pathogens (41.7% vs. 8.3%) [43]. This enhanced detection capability is crucial in populations susceptible to opportunistic infections and co-infections.
Experimental Protocols and Methodologies
Standardized mNGS Workflow
The mNGS workflow consists of multiple critical steps, each requiring optimization to ensure diagnostic accuracy while minimizing contamination and bias.
Standard mNGS Experimental Workflow
Detailed Methodological Protocols
Sample Processing and Nucleic Acid Extraction
The initial phase of mNGS testing requires meticulous sample handling to preserve nucleic acid integrity while maximizing pathogen recovery. For cerebrospinal fluid samples, typical protocols involve processing 600 μL of CSF mixed with enzymes and glass beads, followed by vigorous vortexing at 2,800-3,200 rpm for 30 minutes [47]. Total DNA extraction is then performed using commercial kits such as the TIANamp Micro DNA Kit, with careful attention to minimizing contamination [47] [48]. For bronchoalveolar lavage fluid, additional pretreatment steps may include sterilization at 65°C for 30 minutes and lysozyme treatment to break down bacterial cell walls [41].
The DNA extraction process follows manufacturer recommendations, with DNA concentration measured using fluorescent quantification methods like the Qubit dsDNA HS Assay Kit [41]. This step is critical as insufficient input DNA can lead to failed libraries or inadequate sequencing depth, while excess host DNA can swamp pathogen signals.
Library Preparation and Sequencing
Library construction typically employs transposase-based fragmentation methods (Nextera XT, KAPA HyperPlus) that simultaneously fragment DNA and add sequencing adapters [41] [42]. Following end repair, adapter ligation, and PCR amplification, libraries undergo rigorous quality control using instruments such as the Agilent 2100 Bioanalyzer to assess fragment size distribution and concentration [48].
Sequencing is most commonly performed on Illumina platforms (NextSeq, MiSeq) using single-end or paired-end chemistry, with a target of 20 million reads per sample to ensure sufficient depth for detecting low-abundance pathogens [41] [42]. The BGISEQ platform is also widely used, particularly in Chinese clinical laboratories [48]. Each sequencing run includes negative controls (no-template water or buffer) to monitor for contamination introduced during library preparation or sequencing.
Bioinformatic Analysis Pipeline
The bioinformatic workflow begins with quality control of raw sequencing data using tools like fastp to remove low-quality reads, adapter sequences, and short fragments (<35-50 bp) [46] [42]. Human sequence depletion is then performed by alignment to reference genomes (GRCh38/hg38) using Burrows-Wheeler Aligner or other mapping tools [48] [7].
The remaining non-human reads are classified by alignment to comprehensive microbial databases containing bacterial, viral, fungal, and parasite genomes downloaded from NCBI or other public repositories [47] [48]. Positive detection thresholds vary by pathogen type but typically require reads to be significantly enriched over negative controls (RPMsample/RPMNTC ≥ 5-10) [42] [7]. For specific pathogens with low contamination risk like Mycobacterium tuberculosis, even a single unique read may be considered significant [48].
The Scientist's Toolkit: Essential Research Reagents and Platforms
Successful implementation of mNGS in pathogen discovery requires carefully selected reagents, instruments, and computational resources. The following table details essential components of the mNGS workflow.
Table 3: Essential Research Reagents and Platforms for mNGS Pathogen Discovery
| Category | Specific Products/Platforms | Key Function | Performance Considerations |
|---|---|---|---|
| Nucleic Acid Extraction | TIANamp Micro DNA Kit (Tiangen) [47] [48], QIAamp DNA Micro Kit (Qiagen) [7] | Isolation of microbial nucleic acids from clinical samples | Efficiency varies by pathogen type; critical for robust detection |
| Library Preparation | KAPA HyperPlus Kit (Roche) [42], Nextera XT DNA Library Prep Kit (Illumina) [7], PMseq kits (BGI) [41] | Fragmentation, adapter ligation, and amplification for sequencing | Impact library complexity and representation |
| Sequencing Platforms | Illumina NextSeq/MiSeq [41] [42], BGISEQ-50/MGISEQ-2000 (MGI) [48] | High-throughput sequencing | Different throughput, read length, and error profiles |
| Bioinformatic Tools | Burrows-Wheeler Aligner, Trimmomatic, fastp [42] [7] | Quality control, host depletion, microbial alignment | Critical for sensitive, specific pathogen detection |
| Reference Databases | NCBI RefSeq, in-house curated databases [47] [48] | Taxonomic classification of sequencing reads | Comprehensiveness impacts range of detectable pathogens |
| Quality Control Reagents | Qubit dsDNA HS Assay Kit, Agilent 2100 Bioanalyzer kits [41] [7] | Assessment of nucleic acid and library quality | Essential for troubleshooting and process optimization |
| K-Ras-IN-2 | K-Ras-IN-2 | KRAS Antagonist Research Compound | K-Ras-IN-2 is a potent K-Ras antagonist for cancer research. This product is for research use only (RUO) and not for human use. | Bench Chemicals |
| Acefylline | Acefylline, CAS:652-37-9, MF:C9H10N4O4, MW:238.20 g/mol | Chemical Reagent | Bench Chemicals |
The selection of appropriate reagents and platforms depends on multiple factors, including sample type, target pathogens, and available infrastructure. For instance, the Illumina platform dominates clinical applications with more than 50% of studies using this technology [40], while BGISEQ platforms are widely implemented in Chinese clinical laboratories [41] [48]. Extraction efficiency varies considerably across pathogen types, with gram-positive bacteria, mycobacteria, and fungi presenting particular challenges due to their robust cell walls [42].
Critical Analysis of Strengths and Limitations in Clinical Implementation
Key Advantages of mNGS in Pathogen Discovery
The unbiased nature of mNGS provides several distinct advantages over both culture and targeted molecular methods. Most significantly, mNGS enables detection of unexpected, rare, or novel pathogens without requiring prior suspicion [40] [44]. In a study of patients with severe infections, mNGS exhibited particular strength in identifying rare pathogens such as Chlamydia pneumoniae, Mycobacterium tuberculosis complex, and Legionella pneumophila that were undetectable by conventional methods [41]. This comprehensive detection capability is further enhanced by the ability to identify multiple pathogens in co-infections, which occurred in 27.1% of HIV patients with CNS disorders [43].
Turnaround time represents another significant advantage, with mNGS typically delivering results within 24-72 hours compared to days or weeks for culture [40]. This rapid detection is particularly valuable for slow-growing organisms like Mycobacterium tuberculosis, which requires 2-8 weeks for culture identification [46]. The speed of mNGS facilitates earlier targeted therapy, potentially improving patient outcomes and antimicrobial stewardship.
mNGS also demonstrates superior sensitivity compared to culture methods, especially in patients who have received prior antibiotic therapy [41]. Unlike culture, which depends on viable microorganisms, mNGS detects nucleic acids from both viable and non-viable organisms, increasing detection rates in partially treated infections [40].
Practical Limitations and Diagnostic Challenges
Despite its considerable advantages, mNGS faces several important limitations that affect its clinical implementation. Specificity challenges arise primarily from the detection of environmental contaminants, colonization, and non-pathogenic microbial nucleic acids present in reagents or introduced during sample collection [42]. In pulmonary infection studies, 47.1% of microbial strains initially identified as potential pathogens by mNGS were reclassified as colonizers after clinical correlation [42]. This underscores the necessity for careful interpretation of results within the clinical context.
The high cost of mNGS testing presents a significant barrier to widespread adoption, particularly in resource-limited settings where the burden of infectious diseases is highest [40] [43]. While precise cost figures vary by region and healthcare system, mNGS remains substantially more expensive than conventional culture or targeted PCR.
Analytical sensitivity remains challenging in samples with high host background and low microbial biomass [40] [44]. The extensive human sequence data generated (often >95% of total reads) can obscure pathogen detection, particularly for organisms present at low abundance [44]. This limitation is most pronounced in meningitis and encephalitis cases, where pathogen loads in CSF may be extremely low.
Technical variability in sample processing, sequencing depth, and bioinformatic pipelines further complicates inter-laboratory comparison and standardization [40]. The field currently lacks universal workflow validation and quality assurance protocols, though efforts are underway to establish best practices and proficiency testing programs.
The evidence from multiple clinical studies across diverse infection types demonstrates that mNGS represents a powerful addition to the pathogen discovery toolkit, rather than a wholesale replacement for conventional methods. The technology's unbiased nature, broad detection range, and rapid turnaround time address significant limitations of both culture-based and targeted molecular approaches. However, its optimal implementation requires careful consideration of its limitations, including cost, potential for false positives, and challenges in detecting low-abundance pathogens against high host background.
For researchers and clinical microbiologists, the current evidence supports a complementary diagnostic strategy that leverages the respective strengths of different methodologies. mNGS excels as a first-line test for critically ill patients with diagnostically challenging presentations, as a second-line test when conventional methods yield negative results despite high clinical suspicion, and for detection of fastidious, rare, or novel pathogens [42] [44]. Culture remains essential for antimicrobial susceptibility testing and provides important contextual information for distinguishing colonization from true infection.
As the technology continues to evolve, addressing current challenges related to standardization, cost reduction, and bioinformatic analysis will further solidify mNGS as an indispensable tool in the pathogen discovery arsenal. The ongoing comparison between culture, molecular, and metagenomic methods will undoubtedly continue to refine their respective roles in the diagnostic ecosystem, ultimately advancing our ability to rapidly and accurately identify the microbial causes of infectious diseases.
The field of pathogen discovery research is defined by a critical methodological schism: the long-standing, culture-based paradigm versus modern molecular techniques. Traditional culture methods, while historically the gold standard, are often slow, labor-intensive, and ill-suited for rapid response in public health crises [49]. Molecular methods like polymerase chain reaction (PCR) revolutionized the field with improved speed and sensitivity but frequently require sophisticated thermal cyclers, professional operation, and advanced laboratory environments, limiting their application in resource-limited settings [50] [51]. Against this backdrop, CRISPR-based diagnostic systems have emerged as a transformative force. These systems leverage a bacterial adaptive immune mechanism to create diagnostic tools that are not only rapid and highly precise but also portable and accessible. This guide objectively compares the performance of emerging CRISPR-based diagnostic platforms against traditional and alternative molecular methods, providing researchers and drug development professionals with the experimental data and protocols necessary to critically evaluate this groundbreaking technology.
The core advantage of CRISPR diagnostics lies in their programmability and inherent signal amplification. Systems utilizing Cas proteins such as Cas12, Cas13, and Cas9 can be directed by guide RNA to locate a specific nucleic acid sequence of a pathogen. Upon recognition, some Cas proteins exhibit trans-cleavage activity, non-specifically cutting surrounding reporter molecules to generate a detectable signal [50] [51]. This mechanism allows for unparalleled sensitivity, capable of detecting attomolar (aM) concentrations of a target, and specificity that can distinguish single-nucleotide polymorphisms [50]. This review will dissect the molecular mechanisms of these tools, compare their clinical efficacy with traditional methods using summarized quantitative data, and highlight cutting-edge innovations such as amplification-free detection and AI integration that are pushing the boundaries of diagnostic science.
Comparative Analysis: CRISPR vs. Traditional Diagnostic Methods
To understand the performance characteristics of CRISPR-based diagnostics, it is essential to compare them directly with established methods across key parameters. The following table synthesizes data from multiple experimental studies to provide a clear, quantitative comparison.
Table 1: Performance Comparison of Pathogen Detection Methods
| Method | Detection Time | Sensitivity | Specificity | Equipment Needs | Key Applications |
|---|---|---|---|---|---|
| Culture-Based | 2-7 days [49] | Variable (requires viable pathogen) | High [49] | Incubators, microbiological supplies [49] | Gold standard for viability, antibiotic susceptibility testing [49] |
| PCR/qPCR | 1-4 hours [50] [51] | High (copies/µL) | High [50] | Thermal cycler, skilled personnel [50] [51] | Broad-spectrum nucleic acid detection, quantification (qPCR) |
| CRISPR (e.g., DETECTR, SHERLOCK) | 15 mins - 1 hour [50] [49] | Ultra-high (aM level) [50] | Single-base pair specificity [50] | Minimal (isothermal incubation); portable readers [50] [49] | Point-of-care testing, detection of viruses, bacteria, SNPs [50] [52] |
The data reveals CRISPR's distinct advantages in speed, sensitivity, and operational simplicity. For instance, in detecting foodborne pathogens like Salmonella and E. coli, CRISPR assays have demonstrated a time-to-result of under 4 hours, a significant improvement over the 24-48 hours typically required by culture-based methods [49]. Furthermore, a study on detecting Tomato Brown Rugose Fruit Virus (ToBRFV) showed that a CRISPR-Cas13a assay outperformed RT-PCR, RT-qPCR, and RT-LAMP, successfully detecting the virus at high dilutions (1:200, 1 ng/µL) [53].
Beyond the metrics above, the fundamental difference lies in the ecological adaptability of CRISPR. While traditional methods are largely confined to centralized laboratories, CRISPR diagnostics can be deployed in field settings. This is evidenced by platforms integrated into lyophilized formats, microfluidic microarrays, and lateral flow assays, enabling point-of-care diagnostics with minimal instrumentation [50]. However, challenges remain. The performance of CRISPR assays can be inhibited by complex sample matrices (e.g., food, blood), and scalability for high-throughput clinical use is still being optimized [50] [49].
Molecular Mechanisms and Key CRISPR Platforms
The diagnostic utility of CRISPR systems stems from the distinct biochemical properties of different Cas enzymes. The workflow typically involves two stages: an initial amplification of the target nucleic acid (e.g., using recombinase polymerase amplification, or RPA) to boost sensitivity, followed by the CRISPR-Cas detection and signal generation step [49]. The following diagram illustrates this general workflow for DNA and RNA targets.
General Workflow for CRISPR Diagnostics
Key Diagnostic Cas Enzymes
The following table details the mechanisms of the primary Cas proteins used in diagnostics.
Table 2: Key CRISPR-Cas Proteins and Their Diagnostic Mechanisms
| Cas Protein | Target | PAM/PFS Requirement | Trans-Cleavage Activity | Example Platform |
|---|---|---|---|---|
| Cas9 | DNA | Yes (PAM: 5'-NGG-3') [50] | No (cis-cleavage only) [50] | Used for target enrichment and FISH-based detection [51] |
| Cas12 (e.g., Cas12a) | DNA | Yes (T-rich PAM) [50] | Yes (collateral cleavage of ssDNA) [50] [51] | DETECTR [50] |
| Cas13 (e.g., Cas13a) | RNA | Yes (PFS: 3' of target) [50] | Yes (collateral cleavage of ssRNA) [50] [51] | SHERLOCK [50] |
The collateral cleavage activity of Cas12 and Cas13 is the cornerstone of modern CRISPR diagnostics. When these proteins find and bind to their target sequence, they become activated to indiscriminately cleave nearby reporter molecules. These reporters are typically nucleic acids (ssDNA for Cas12, ssRNA for Cas13) linked to a fluorophore and a quencher. Cleavage separates the fluorophore from the quencher, generating a fluorescent signal that can be detected with a portable reader or even visually in some cases [50] [51]. This mechanism provides a massive signal amplification from a single target recognition event.
Experimental Protocols and Workflows
To illustrate the practical application of these mechanisms, this section details a standard experimental protocol for a Cas12-based detection assay (modeled on the DETECTR platform) and an innovative AI-enhanced workflow.
Standard Cas12a DETECTR Protocol for DNA Virus Detection
This protocol is adapted from methods used for detecting human pathogens like HPV and Zika virus [50] [51], as well as foodborne pathogens [49].
Table 3: Key Research Reagent Solutions for DETECTR Assay
| Reagent / Material | Function / Description | Example Vendor / Note |
|---|---|---|
| LbCas12a Protein | The CRISPR effector enzyme that provides target-specific recognition and collateral cleavage activity. | Can be recombinantly expressed and purified or purchased from commercial enzyme suppliers. |
| crRNA (for LbCas12a) | Guide RNA specific to the target DNA sequence (e.g., a conserved region of the viral genome). | Synthesized in vitro; design requires a TTTV PAM sequence adjacent to the target site. |
| ssDNA Fluorescent Reporter | A molecule (e.g., 6-FAM/TTATT/3BHQ-1) that is cleaved by activated Cas12a, producing a fluorescent signal. | Available from oligonucleotide synthesis companies; quencher and fluorophore selection is customizable. |
| Recombinase Polymerase Amplification (RPA) Kit | Isothermal amplification kit to pre-amplify the target DNA, enhancing assay sensitivity. | Commercially available kits from companies like TwistDx. |
| Plate Reader or Lateral Flow Strips | Detection device. Fluorescent output can be measured quantitatively, or adapted for lateral flow readouts. | Various manufacturers for microplate readers; lateral flow strips from diagnostic suppliers. |
Procedure:
- Nucleic Acid Extraction: Extract total nucleic acid from the sample (e.g., patient swab, food homogenate) using a commercial kit.
- Target Pre-amplification: Perform an RPA reaction on the extracted DNA.
- Reaction Mix: Combine RPA rehydration buffer, forward and reverse primers (specific to the target), template DNA, and nuclease-free water. Initiate the reaction by adding magnesium acetate.
- Incubation: Incubate at 37-42°C for 15-20 minutes.
- CRISPR-Cas12a Detection:
- Prepare the detection mix containing:
- LbCas12a nuclease (e.g., 50 nM final concentration)
- Target-specific crRNA (e.g., 50 nM final concentration)
- ssDNA fluorescent reporter (e.g., 500 nM final concentration)
- Appropriate reaction buffer
- Combine the detection mix with a portion of the RPA amplification product.
- Incubate the reaction at 37°C for 5-15 minutes.
- Prepare the detection mix containing:
- Signal Detection:
- Fluorescence Measurement: Transfer the reaction to a plate reader and measure the fluorescence intensity at the relevant wavelength (e.g., excitation/emission for FAM). A significant increase over a negative control indicates a positive result.
- Lateral Flow Readout: As an alternative, the reaction can be applied to a lateral flow strip, where cleavage of a labeled reporter produces a visible test line.
Advanced Protocol: AI-Integrated CRISPR-Cas13a Pipeline
Recent innovations have integrated machine learning to optimize guide RNA design, dramatically improving the specificity and development speed of CRISPR diagnostics. The following diagram and protocol are based on a 2025 study for detecting Tomato brown rugose fruit virus (ToBRFV), a model applicable to human RNA viruses [53].
AI-Driven CRISPR Diagnostic Development
Procedure:
- Computational crRNA Design:
- Sequence Retrieval & Alignment: Automatically retrieve target virus genome sequences from NCBI GenBank using a Python script with the Biopython Entrez module. Perform a multiple sequence alignment (MSA) using Clustal Omega.
- Consensus Generation: Generate a consensus sequence from the MSA using the Majority Rule Algorithm, excluding positions with less than 70% conservation.
- crRNA Candidate Selection: Use a sliding window (23-28 nt) to generate all possible crRNAs from the consensus. Filter candidates based on GC content (40-60%), absence of long homopolymers (≥4 identical nucleotides), and seed region sequence.
- Machine Learning Evaluation: Train a Random Forest Classifier on a synthetic dataset of sequences labeled by GC content rules. Use the model to predict the effectiveness of candidate crRNAs.
- Off-Target Analysis: Screen the final candidate crRNAs against the host genome (e.g., human, tomato) using the fuzzysearch library, allowing for up to 3 mismatches to identify and eliminate designs with high off-target potential.
- Experimental Validation:
- Synthesize the top-ranked crRNA candidates from the computational pipeline.
- Perform a fluorescence-based Cas13a assay as described in the standard protocol, but using the designed crRNA, Cas13a protein, and an RNA target.
- Compare the detection sensitivity and specificity of the AI-designed crRNAs against established methods like RT-qPCR and RT-LAMP through serial dilution experiments of the target RNA [53]. The published study demonstrated that this pipeline produced crRNAs enabling detection superior to these conventional methods [53].
CRISPR-based diagnostic systems represent a paradigm shift in pathogen detection, effectively bridging the gap between the high precision of molecular methods and the practical needs of point-of-care testing. The experimental data and protocols detailed in this guide demonstrate that platforms like Cas12-DETECTR and Cas13-SHERLOCK consistently outperform traditional culture and PCR-based methods in speed and sensitivity while offering unprecedented specificity and portability [50] [49] [53]. The integration of AI and machine learning for crRNA design further enhances the robustness and scalability of these systems, paving the way for their rapid deployment against emerging pathogens [53].
The future of CRISPR diagnostics is directed toward greater integration and accessibility. Key emerging trends include the development of multiplexed assays capable of detecting dozens of pathogens simultaneously, amplification-free detection to simplify workflows and reduce costs, and the creation of fully automated "sample-to-answer" devices for use in non-laboratory settings [50] [52]. While challenges related to sample preparation, regulatory approval, and ecological adaptability in resource-limited settings remain, the trajectory is clear. As the technology matures, CRISPR-based diagnostics are poised to move from the cutting edge to the cornerstone of a more responsive, equitable, and precise global pathogen surveillance network, fundamentally reshaping the culture of diagnostic research and public health response.
The field of clinical microbiology is undergoing a transformative shift from traditional culture-based methods toward advanced molecular techniques for pathogen discovery. This evolution is particularly critical in time-sensitive scenarios such as central nervous system (CNS) infections, sepsis, and respiratory outbreaks, where rapid and accurate pathogen identification directly impacts patient outcomes and public health responses. Culture methods, long considered the gold standard, provide valuable information about viable pathogens and antimicrobial susceptibility but are limited by prolonged turnaround times (often 24-72 hours) and low sensitivity, especially in patients who have received prior antimicrobial therapy [54] [55]. Molecular methods, including polymerase chain reaction (PCR), metagenomic next-generation sequencing (mNGS), and droplet digital PCR (ddPCR), offer rapid, sensitive, and specific alternatives that are revolutionizing diagnostic pathways.
This guide examines the comparative performance of these methodologies through actual clinical case studies and experimental data, providing researchers and clinicians with evidence-based insights for selecting appropriate diagnostic approaches based on clinical context, available resources, and information needs.
CNS Infections: Diagnostic Challenges and Molecular Solutions
Clinical Case Presentations
Case 1: Recurrent Aseptic Meningitis A 56-year-old male presented with fever, chills, neck stiffness, and worsening headache. Cerebrospinal fluid (CSF) analysis showed 154 white blood cells/mm³ (77% neutrophils), glucose of 63 mg/dL, and protein of 82 mg/dL. Traditional cultures (bacterial, viral, fungal) were negative. Herpes simplex virus (HSV) PCR was negative, but enterovirus RNA PCR returned positive, allowing for targeted supportive care and avoidance of unnecessary antibiotics [54].
Case 2: Encephalitis in an Immunocompromised Host A 72-year-old man on immunosuppressive therapy presented with fatigue, mental status changes, and headache. CSF analysis revealed 120 white blood cells/mm³ (64% lymphocytes). While initial broad-spectrum antimicrobials were administered, Varicella Zoster Virus (VZV) PCR positivity directed specific antiviral therapy with acyclovir, leading to full neurological recovery [54].
Case 3: Herpes Simplex Encephalitis with Sequelae An 86-year-old female presented with 5 days of mental status changes, word-finding difficulties, and gait instability. Brain MRI revealed abnormal signal in the left temporal lobe. CSF PCR for HSV-1 was positive. Despite a 21-day course of IV acyclovir, the patient had residual expressive aphasia and memory deficits at 6 months, highlighting the critical importance of early detection and treatment [54].
Comparative Method Performance in CNS Infections
Table 1: Comparison of Diagnostic Methods for Viral CNS Infections
| Diagnostic Method | Time to Result | Key Advantages | Key Limitations | Clinical Impact |
|---|---|---|---|---|
| CSF Culture | 3-7 days | Assess viability, antimicrobial susceptibility | Low sensitivity for viruses; too slow for acute management | Limited for viral diagnosis |
| CSF PCR | 2-6 hours | High sensitivity (90-95%) and specificity; rapid | Targeted (requires clinical suspicion); limited multiplexing | Directs specific antiviral therapy; enables antibiotic stewardship |
| mNGS | 2-3 days | Unbiased detection; novel pathogen discovery | Cost; bioinformatics complexity; interpretation challenges | Pathogen discovery in enigmatic cases |
The utility of molecular diagnostics is particularly evident in viral CNS infections, where the combined use of CSF PCR and clinical presentation has become the diagnostic standard. In the cases above, PCR provided results within hours, enabling rapid discontinuation of unnecessary antibacterial agents in Cases 1 and 2, and appropriate antiviral therapy in all three cases [54].
Sepsis and Bloodstream Infections: The Race Against Time
Experimental Comparison of Detection Methods
A 2021 prospective study directly compared three diagnostic methods for bloodstream infections in 60 critically ill patients with suspected sepsis [55]:
- Blood Culture (gold standard): 10/60 positive (16.7%)
- Droplet Digital PCR (ddPCR): 50/60 positive (83.3%)
- Metagenomic NGS (mNGS): 41/60 positive (68.3%, excluding viruses)
Table 2: Head-to-Head Comparison of BSI Detection Technologies
| Parameter | Blood Culture | Droplet Digital PCR | Metagenomic NGS |
|---|---|---|---|
| Overall Sensitivity | 16.7% | 83.3% | 68.3% (for bacteria/fungi) |
| Turnaround Time | 2-5 days | ~4 hours | ~2 days |
| Pathogen Range | Limited to cultivable organisms | 20 predefined pathogens + 4 AMR genes | Broad, untargeted detection (including viruses) |
| AMR Detection | Via AST (additional 24-48h) | Direct (mecA, blaKPC, etc.) | Possible but complex (requires sufficient coverage) |
| Key Strength | Gold standard; provides live isolates | Speed + sensitivity for predefined targets | Unbiased pathogen discovery |
| Major Limitation | Low sensitivity; slow | Limited target range | Cost; technical complexity |
Among the 10 culture-positive episodes, both ddPCR and mNGS concordantly identified 9 (90%), demonstrating excellent correlation with culture when it is positive. However, the molecular methods detected significantly more pathogens, likely due to the low microbial inoculum or prior antibiotic administration that often inhibits cultural growth [55].
Sepsis Identification Algorithms and Their Impact
Beyond pathogen detection, defining sepsis itself varies substantially by methodology. A retrospective cohort study of 11,791 ICU admissions demonstrated how different identification criteria capture distinct patient populations [56]:
Table 3: Comparison of Sepsis Identification Criteria and Outcomes
| Identification Method | Sepsis Incidence | In-Hospital Mortality | Key Characteristics |
|---|---|---|---|
| Sepsis-3 (SOFA ≥2 + infection) | 49.1% | 14.5% | Larger, less severely ill cohort |
| CDC Surveillance Criteria | 31.9% | 22.1% | Similar infection suspicion to Sepsis-3 |
| Angus Methodology (ICD-9) | 28.6% | 24.8% | Administrative coding-based |
| Explicit Sepsis Codes | 9.0% | 30.1% | Smallest, most severely ill cohort |
The Sepsis-3 criteria (organ dysfunction defined by SOFA score ≥2 points plus suspected infection) identified a larger, less severely ill cohort compared to administrative code-based methods, which captured smaller populations with higher mortality rates [56].
Respiratory Outbreaks: Syndromic Surveillance and Molecular Tools
Contemporary Surveillance Frameworks
Respiratory virus surveillance has evolved from influenza-like illness (ILI) tracking to broader acute respiratory illness (ARI) surveillance, capturing a wider spectrum of respiratory pathogens beyond influenza. The CDC's ARI metric categorizes emergency department visits into five activity levels (Very Low to Very High) based on deviations from baseline respiratory illness activity [57].
The wastewater-based epidemiology (WBE) has emerged as a powerful complementary tool, providing early warning of community transmission 1-2 weeks before clinical case surges. As of late 2025, WBE platforms monitor SARS-CoV-2, influenza A, and RSV in wastewater, offering unique insights into infection dynamics, including presymptomatic and asymptomatic transmission [58] [57].
Diagnostic Methodologies in Respiratory Infections
Molecular methods for respiratory pathogen detection span multiple technological approaches:
- PCR-based platforms: Remain the workhorse for clinical diagnostics with rapid turnaround (hours)
- Multiplex panels: Simultaneously detect 20+ respiratory pathogens from single specimens
- NGS approaches: Enable unbiased pathogen discovery and variant characterization
- Wastewater analysis: Utilizes qPCR, ddPCR, and NGS for community-level surveillance
The pandemic and epidemic-prone diseases framework employed by organizations like PAHO emphasizes integrated surveillance combining epidemiology, modeling, laboratory networks, and clinical management to create a comprehensive respiratory threat response system [59].
Experimental Protocols and Methodologies
Bloodstream Infection Detection Workflow
Experimental protocol for comparative BSI detection from [55]:
- Sample Collection: Simultaneous draw of whole blood for culture, ddPCR, and mNGS upon clinical suspicion of BSI
- Blood Culture: 2 sets (aerobic/anaerobic) incubated in BacT/ALERT 3D System; positive cultures identified by MALDI-TOF MS
- ddPCR Protocol:
- Plasma separation within 1 hour of collection
- DNA extraction using magnetic bead-based kits
- ddPCR reaction setup with pathogen-specific assays
- Droplet generation and PCR amplification
- Quantification of target molecules
- mNGS Protocol:
- DNA extraction from 200μL plasma
- Library preparation with dual-indexing
- Sequencing on Illumina platforms (20-30 million reads)
- Bioinformatic analysis with human sequence subtraction
- Microbial classification and reporting
Sepsis Algorithm Validation Methodology
Validation approach for sepsis identification methods from [56]:
- Cohort Definition: 11,791 adult ICU admissions (2008-2012) from MIMIC-III database
- Suspected Infection Criteria: Blood culture collection + antibiotic administration within 24h window
- Organ Dysfunction Assessment: SOFA score calculation using worst values in 24h before/after suspicion
- Comparator Algorithms:
- Explicit ICD-9 codes (995.92, 785.52)
- Angus implementation (ICD-9 codes for infection + organ dysfunction)
- Martin criteria (ICD-9 codes for bacteremia + organ dysfunction)
- CMS SEP-1 criteria (combination of codes, SIRS, organ dysfunction)
- CDC surveillance criteria (similar infection suspicion with organ dysfunction)
- Statistical Analysis: Discrimination for hospital mortality using AUROC; agreement statistics
Visualization of Diagnostic Pathways and Workflows
Diagnostic Pathway for CNS Infections
BSI Detection Technology Comparison
The Scientist's Toolkit: Essential Research Reagents and Solutions
Table 4: Key Research Reagents and Solutions for Pathogen Detection Studies
| Reagent/Solution | Application | Function/Purpose | Example from Studies |
|---|---|---|---|
| BacT/ALERT Culture Media | Blood culture | Supports growth of aerobic/anaerobic microorganisms | BacT/ALERT 3D System for BSI detection [55] |
| Magnetic Serum/Plasma DNA Kits | Nucleic acid extraction | Isolate microbial DNA from clinical samples | Auto-Pure20B System for ddPCR/mNGS [55] |
| Pathogen-Specific Primer/Probe Sets | ddPCR/qPCR | Amplify target pathogen sequences | 20-plex pathogen panel for BSI [55] |
| DNA Library Prep Kits | mNGS | Fragment, index, and prepare DNA for sequencing | Illumina-compatible kits for plasma mNGS [55] |
| SOFA Score Parameters | Sepsis identification | Quantify organ dysfunction for sepsis criteria | Respiratory, coagulation, liver, cardiovascular, CNS, renal assessment [56] |
| Bioinformatic Pipelines | mNGS analysis | Subtract human sequences, classify microbial reads | Kraken, Metaphlan for taxonomic classification [55] |
The evidence from these case studies and comparative analyses demonstrates that modern pathogen detection relies on a complementary approach rather than exclusive reliance on any single methodology. Culture methods remain essential for antimicrobial susceptibility testing and provide the reference standard, but molecular techniques offer transformative advantages in speed, sensitivity, and scope of detection.
In CNS infections, CSF PCR has become first-line testing for common viral pathogens, while mNGS reserves its value for enigmatic cases. In sepsis, ddPCR provides unprecedented speed and sensitivity for predefined targets, while mNGS offers unparalleled breadth for pathogen discovery. For respiratory outbreaks, syndromic surveillance integrated with wastewater monitoring provides early warning systems that complement clinical diagnostics.
The evolving diagnostic landscape suggests that future advancements will focus on integrating multiple methodologies, developing faster sample-to-answer systems, and improving bioinformatic interpretation to maximize clinical utility. As these technologies continue to mature, the optimal approach will balance speed, sensitivity, cost, and actionable results to improve patient outcomes across the spectrum of infectious diseases.
Navigating Diagnostic Challenges: Sensitivity, Specificity, and Practical Hurdles
The shift from traditional culture-based methods to molecular diagnostics represents a paradigm shift in pathogen discovery research. While culture-independent diagnostic tests (CIDTs) provide unprecedented speed and sensitivity, they introduce a significant interpretive challenge: determining whether a positive signal indicates an active, clinically relevant infection or merely the presence of non-viable genetic residue from dead microorganisms [60] [61]. This distinction is not merely academic; it directly impacts patient treatment, antibiotic stewardship, and public health surveillance. The persistence of detectable DNA after pathogen death can lead to false-positive results, potentially triggering unnecessary treatments and contributing to antimicrobial resistance [61]. Consequently, researchers and clinicians must understand the technical nuances and limitations of both established and emerging diagnostic platforms to accurately interpret their results.
This guide objectively compares the performance of culture-based and molecular methods, with particular focus on their ability to differentiate active infection from genetic residue. We present experimental data and methodological details to equip researchers and drug development professionals with the analytical framework needed to navigate this complex diagnostic landscape.
Comparative Performance Data: Culture vs. Molecular Methods
Detection Sensitivity and Turnaround Time
Table 1: Comparison of key performance metrics between diagnostic methods.
| Method Category | Specific Technology | Reported Sensitivity | Typical Turnaround Time | Ability to Distinguish Viable Pathogens |
|---|---|---|---|---|
| Culture-Based | Standard blood culture | Reference standard | 15 hours - 5 days [30] [62] | High (detects only viable, replicating organisms) |
| Molecular | Broad-range PCR (16S rRNA) | Higher than culture in multiple studies [63] [64] | ~2-6 hours [61] [62] | Low (detects DNA from both live and dead cells) [61] |
| Molecular | Digital DNA Melting Analysis | Matches culture with faster results [62] | <6 hours [62] | High (claims to detect only intact organisms) [62] |
| Molecular | mNGS | 86.6% vs. 59.1% for culture in NCNSIs [64] | ~16.8 hours [64] | Low (detects nucleic acids without viability context) |
| Molecular | ddPCR | 78.7% vs. 59.1% for culture in NCNSIs [64] | ~12.4 hours [64] | Low (detects nucleic acids without viability context) |
| Molecular | Culture-enriched metagenomic sequencing (CEMS) | Captures 36.5% of species missed by other methods [65] | Several days (includes culture step) | High (combines viability through culture with molecular identification) |
Clinical Performance in Various Infection Types
Table 2: Method performance across different clinical specimen types.
| Infection/Specimen Type | Culture Performance | Molecular Method Performance | Key Comparative Findings |
|---|---|---|---|
| Necrotizing Soft Tissue Infections (NSTIs) | Identified microorganisms in 70% of surgical samples [16] | Identified microorganisms in 90% of samples [16] | Molecular methods detected additional microorganisms, including fungi and fastidious bacteria |
| Polymicrobial Urinary Tract Infections | Detected 22% of polymicrobial infections [60] | PCR detected 95% of polymicrobial infections [60] | PCR revealed polymicrobial infections in 67 patients with negative culture results |
| Ocular Infections | Current gold standard, but limited sensitivity in low-volume specimens [63] | Real-time PCR demonstrated higher sensitivity than conventional PCR [63] | Specimen processing delays significantly affected PCR accuracy |
| Neurosurgical CNS Infections | 59.1% detection rate [64] | mNGS: 86.6%; ddPCR: 78.7% detection rate [64] | mNGS and ddPCR maintained detection despite empiric antibiotics |
| Gastroenteritis (Campylobacter) | 51.2% sensitivity compared to PCR [60] | Significantly superior sensitivity, specificity, and positive predictive value [60] | Culture difficulties due to fastidious growth requirements |
Experimental Protocols for Method Validation
Digital DNA Melting Analysis Protocol
Digital DNA Melting Analysis addresses the viability question by claiming to detect only "intact organisms" rather than free-floating DNA [62]. The experimental workflow involves:
- Sample Preparation: 1 mL of blood is collected in standard blood collection tubes.
- DNA Isolation: Optimized to reduce or eliminate signals from human DNA compared to pathogen DNA, addressing the "needle in a haystack" challenge of pathogen detection in clinical samples [62].
- Digital Amplification: DNA is partitioned and amplified to create thousands of individual reactions.
- High-Resolution Melting: DNA is heated from 50° to 90°C while monitoring fluorescence. The DNA double-helix melts at temperatures dependent on sequence-specific bond strengths, creating unique fingerprints for each pathogen [62].
- Machine Learning Analysis: A specialized algorithm analyzes melt curves against a database of known DNA melt curves, capable of detecting curves from organisms not in the database [62].
This method's key advantage is producing results in under six hours with no reported false positives in a pilot study, compared to 15 hours to several days for culture [62].
Culture-Enriched Metagenomic Sequencing (CEMS) Protocol
A 2025 study developed CEMS to bridge the gap between culture and molecular methods [65]:
- Multi-Media Cultivation: Fresh fecal samples were cultured using 12 commercial or modified media.
- Dual Incubation: Plates were incubated both anaerobically and aerobically at 37°C for 5-7 days.
- Sample Processing: All colonies from each medium were collected by scraping plate surfaces into saline solution.
- DNA Extraction and Sequencing: Metagenomic DNA was extracted from the pooled bacterial cultures and subjected to shotgun metagenomic sequencing using Illumina HiSeq 2500 [65].
- Data Analysis: Sequencing data were analyzed to identify microbiota and calculate growth rate indices (GRiD) to predict optimal media for specific bacteria.
This hybrid approach demonstrated that CEMS and direct culture-independent metagenomic sequencing (CIMS) showed low overlap (18% of species), with each method uniquely identifying 36.5% and 45.5% of species respectively [65].
Broad-Range Real-Time PCR for Ocular Pathogens Protocol
A 2025 study comparing molecular and culture methods for ocular infections established:
- Specimen Collection: Ocular specimens were classified as septic (cornea, conjunctiva) or aseptic (vitreous humor, aqueous humor).
- Nucleic Acid Extraction: Using QIAamp DSP DNA Mini Kit on QIAcube system.
- PCR Setup:
- Conventional PCR: 16 μL reaction volume with 3 μL DNA and 0.4 μM of each primer
- Real-time PCR: 50 μL reaction volume with 4 μL DNA, 0.5 μM primers, and 0.25 μM probe
- Result Interpretation:
- Conventional PCR: Visual confirmation of ~1300 bp amplified band
- Real-time PCR: ΔCT cutoff values determined by ROC analysis (-2.13 for septic, -4.09 for aseptic specimens) [63]
This study highlighted that delays in specimen processing significantly affected real-time PCR accuracy, emphasizing the importance of standardized protocols [63].
Visualizing Methodologies and Workflows
Research Reagent Solutions for Experimental Studies
Table 3: Essential research reagents and their applications in pathogen detection studies.
| Reagent/Kit | Specific Application | Function in Experimental Protocol |
|---|---|---|
| QIAamp DSP DNA Mini Kit (Qiagen) | Nucleic acid extraction from ocular specimens, CSF, and bacterial cultures [63] | High-quality DNA extraction for downstream molecular applications |
| TIANamp Bacteria DNA Kit (Qiagen) | DNA extraction from single-bacterium isolated strains [65] | Prepares template for 16S rRNA gene sequencing and identification |
| QIAamp Fast DNA Stool Mini Kit (Qiagen) | Metagenomic DNA extraction from bacterial cultures and stool samples [65] | Efficient DNA extraction from complex biological matrices |
| Benzonase (Sigma) | Host depletion in mNGS protocols [64] | Removes host nucleic acids to improve pathogen detection sensitivity |
| Various commercial media (LGAM, PYG, GLB, etc.) | Cultivation of intestinal bacteria under diverse conditions [65] | Supports growth of fastidious organisms for CEMS approaches |
| Selective media (MRS-L, RG) | Selective cultivation of Bifidobacterium and Lactobacillus [65] | Enriches for specific bacterial groups to improve detection |
| Blood agar plates, thioglycolate broth | Routine culture of ocular specimens [63] | Standard media for clinical culture-based pathogen detection |
The comparison between culture and molecular methods reveals a nuanced landscape where no single approach provides a perfect solution. Culture methods maintain their essential role in determining microbial viability and providing isolates for antimicrobial susceptibility testing [66] [60]. Conversely, molecular methods offer superior speed and sensitivity, particularly for fastidious or uncultivable pathogens, but struggle to distinguish active infection from genetic residue [61].
Emerging technologies like digital DNA melting analysis and hybrid approaches such as CEMS show promise in bridging this fundamental gap [65] [62]. For researchers and drug development professionals, the optimal path forward involves strategic method selection based on clinical context, and when possible, utilizing complementary approaches that leverage the strengths of both traditional and novel platforms. As molecular technologies continue to evolve, the critical challenge remains developing innovative solutions that preserve the speed and sensitivity of molecular diagnostics while providing the viability context essential for accurate clinical decision-making.
The accurate detection of pathogens is critical in clinical diagnostics and environmental monitoring, yet complex sample matrices such as blood and wastewater present significant technical challenges. These matrices contain inherent inhibitors that can impede molecular and culture-based assays, leading to false negatives and compromised data. This guide objectively compares the performance of traditional microbial culture, metagenomic next-generation sequencing (mNGS), and droplet digital PCR (ddPCR) in these challenging environments. Framed within the broader thesis of comparison culture versus molecular methods in pathogen discovery, we provide experimental data and protocols to inform method selection by researchers, scientists, and drug development professionals. The data reveal a clear trade-off: while molecular methods offer superior speed and sensitivity, culture remains essential for phenotypic analysis, guiding the need for integrated, context-dependent application.
The detection of pathogens in complex samples is a cornerstone of public health, clinical microbiology, and environmental science. However, samples like blood and wastewater are not clean templates; they are complex chemical milieus containing substances that can inhibit the very tests designed to find pathogens. Blood contains heme, immunoglobulins, and anticoagulants, while wastewater is rich in humic acids, heavy metals, and organic pollutants [67] [68]. These compounds can disrupt enzyme function in PCR, impede microbial growth in culture, and introduce biases in sequencing libraries.
This challenge forces a critical methodological choice. Traditional culture methods, the long-standing gold standard, provide vital viability and antibiotic susceptibility data but are slow, often low in sensitivity, and can be completely defeated by prior antibiotic use or unculturable organisms [64]. Molecular methods, including mNGS and ddPCR, offer a rapid, culture-independent alternative with the potential for exquisite sensitivity and comprehensive pathogen identification. This guide directly compares these paradigms, providing a quantitative framework to navigate their respective pitfalls and advantages in the face of pervasive inhibitors.
Methodological Comparison: Core Technologies Head-to-Head
Experimental Protocols & workflows
The following workflows detail the standard operating procedures for each of the three key pathogen detection methods, highlighting where inhibitory compounds in complex matrices can disrupt the process.
Microbial Culture Protocol:
- Sample Collection & Inoculation: Cerebrospinal fluid (CSF) or blood is aseptically collected and directly inoculated into automated culture bottles (e.g., BACTEC) or onto solid agar plates [64].
- Incubation: Inoculated media are placed in incubators at 35-37°C for 24-48 hours, or longer for fastidious organisms. The time to positive culture (TTPC) is a key metric, averaging 15.1 ± 10.4 hours in clinical studies [64].
- Pitfall Point: The presence of empiric antibiotics in the sample is a major inhibitor, preventing the growth of viable organisms and leading to false negatives.
- Identification & Sensitivity Testing: Once growth is detected, colonies are identified using techniques like MALDI-TOF mass spectrometry, and antibiotic susceptibility testing (AST) is performed, which can take an additional 22.6 ± 9.4 hours from sample harvest [64].
Metagenomic Next-Generation Sequencing (mNGS) Protocol:
- Sample Processing & Host Depletion: A 1 mL sample (CSF, blood plasma) is centrifuged to pellet cells and potential pathogens. The pellet is treated with enzymes like Benzonase and detergents (e.g., 0.5% Tween 20) to digest host nucleic acids and liberate pathogen genetic material, a critical step for improving the signal-to-noise ratio [64].
- Pitfall Point: Incomplete host depletion or carryover of enzyme inhibitors from the sample matrix (e.g., heparin from blood, humic acids from wastewater) can inhibit subsequent enzymatic steps.
- Library Preparation & Sequencing: DNA is extracted, and libraries are constructed via fragmentation, adapter ligation, and amplification. Libraries are sequenced on a high-throughput platform (e.g., Illumina) [64].
- Bioinformatic Analysis: Sequences are quality-filtered and aligned against human and microbial databases to identify pathogenic organisms.
Droplet Digital PCR (ddPCR) Protocol:
- Sample Partitioning: A PCR reaction mixture containing the sample DNA, primers, probes, and master mix is partitioned into ~20,000 nanoliter-sized droplets [64].
- Pitfall Point: Particulate matter or viscous components in crude samples can clog microfluidic channels and prevent successful droplet generation.
- Endpoint PCR Amplification: The droplets undergo a standard PCR thermal cycling process. Each droplet acts as an individual reaction vessel.
- Droplet Reading & Quantification: A droplet reader counts the number of fluorescence-positive (containing the target sequence) and negative droplets. Using Poisson statistics, the absolute concentration of the target nucleic acid in the original sample is calculated without the need for a standard curve [64].
Research Reagent Solutions
The following table details key reagents and materials essential for overcoming inhibition in complex sample analysis.
Table 1: Essential Research Reagents for Pathogen Detection in Complex Matrices
| Item | Function | Application Notes |
|---|---|---|
| Benzonase | Enzyme that degrades host and free-floating nucleic acids. | Critical for mNGS host depletion to increase microbial sequencing depth [64]. |
| PFAS-Specific Resin | Ion exchange resin with hydrophobic polymer backbone and charged functional groups. | Used in wastewater treatment to adsorb PFAS via electrostatic and hydrophobic interactions, removing these potent inhibitors [69]. |
| ddPCR Supermix | Optimized PCR reaction mix resistant to common inhibitors. | Formulated to maintain polymerase activity in the presence of humic acids, heparin, and other contaminants [64]. |
| Microbial Culture Bottles | Liquid media with sensors for automated growth detection. | Contains resins to neutralize certain antibiotics, mitigating this key inhibitor for culture [64]. |
| Magnesium-Based Reagent | A "green" neutralizing reagent used in situ. | Precipitates metals and neutralizes acidity in wastewater, reducing matrix interference for subsequent analysis [67]. |
Performance Data & Comparative Analysis
Quantitative Performance Metrics
Direct comparison of experimental data from a clinical study of 127 patients with neurosurgical central nervous system infections (NCNSIs) reveals stark differences in method performance [64].
Table 2: Comparative Diagnostic Performance of Pathogen Detection Methods
| Parameter | Microbial Culture | mNGS | ddPCR |
|---|---|---|---|
| Positive Detection Rate | 59.1% | 86.6% (p<0.01) | 78.7% (p<0.01) |
| Impact of Empiric Antibiotics | Significant reduction in sensitivity | Minimal impact | Minimal impact |
| Mean Time to Result (Hours) | 22.6 ± 9.4 | 16.8 ± 2.4 | 12.4 ± 3.8 |
| Detection of Rare/Novel Pathogens | Limited to culturable species | Excellent | Poor (requires prior target knowledge) |
| Quantification Capability | Semi-quantitative | Semi-quantitative (with controls) | Absolute |
Analysis of Inhibitor Susceptibility
The data in Table 2 underscores a fundamental weakness of culture methods: vulnerability to pre-analytical factors like antibiotic administration. In the cited study, 29.1% of patients (37/127) were positive by mNGS but negative by culture, a discrepancy largely attributed to empiric antibiotic use [64]. Molecular methods bypass this limitation by detecting genetic material rather than relying on viable organisms.
Furthermore, the speed advantage of molecular methods is critical for acute decision-making. ddPCR provided results significantly faster than both mNGS and culture (p<0.01) [64]. However, each molecular method has its own niche; mNGS is superior for hypothesis-free detection of unknown or mixed infections, while ddPCR excels at rapid, sensitive, and absolute quantification of a predefined target, even in the presence of PCR inhibitors due to its partitioning technology [64].
In environmental contexts like wastewater, similar principles apply. Technologies like advanced oxidation processes (AOPs) and membrane bioreactors (MBRs) must contend with fluctuating loads and competing background compounds that inhibit treatment efficiency [68] [69]. For instance, the presence of natural organic matter (NOM) can compete with target pollutants like PFAS for active sites on ion-exchange resins, reducing their removal efficiency [69].
Visualizing Workflows and Logical Pathways
Pathogen Detection Method Selection
Inhibitor Effects on Molecular Workflows
The confrontation with inhibitors in complex matrices reveals that no single pathogen detection method is universally superior. The choice between culture, mNGS, and ddPCR is dictated by the specific clinical or research question. Culture remains indispensable for phenotypic confirmation and AST, molecular methods have shattered the barriers of speed, sensitivity, and culturability. The data clearly show that mNGS and ddPCR significantly outperform culture in detection rates and turnaround time, particularly in the presence of antibiotics [64]. The future of pathogen discovery lies not in the supremacy of one paradigm over the other, but in their strategic integration, using molecular methods for rapid screening and identification, and refining culture for definitive phenotypic characterization. This synergistic approach, mindful of the technical pitfalls in each matrix, will most effectively combat the challenges of inhibitor-rich environments and advance both diagnostic medicine and environmental science.
The field of clinical microbiology is undergoing a fundamental transformation, moving from a traditional "comparison culture" reliant on phenotypic methods toward sophisticated molecular techniques for pathogen identification. This evolution represents more than a simple technological upgrade; it signifies a fundamental shift in diagnostic philosophy, workflow, and resource allocation. Historically, microbial culture has served as the gold standard, providing a phenotypic basis for identification and antibiotic susceptibility testing [66]. However, this method is time-consuming, with growth periods ranging from hours to days, and its sensitivity is frequently compromised by prior antibiotic administration and the presence of fastidious organisms [16] [64]. The emergence of molecular methods—including quantitative PCR (qPCR), droplet digital PCR (ddPCR), and next-generation sequencing (mNGS and tNGS)—offers a powerful, genotype-based alternative. These techniques provide rapid, sensitive, and often comprehensive pathogen detection, but their implementation is fraught with significant financial and infrastructural challenges, particularly in low-resource settings. This guide objectively compares the performance of these diagnostic pathways and explores the practical and economic barriers to their adoption.
Performance Comparison: Molecular Methods vs. Traditional Culture
Extensive research has quantified the performance differences between traditional and molecular diagnostic techniques. The following tables summarize key comparative data across different infection types and methodological approaches.
Table 1: Overall Pathogen Detection Performance Across Sample Types
| Infection Type / Study | Traditional Culture Detection Rate | Molecular Method Detection Rate | Statistical Significance (p-value) | Key Molecular Advantages |
|---|---|---|---|---|
| Neurosurgical CNS Infections (n=127) [64] | 59.1% | mNGS: 86.6%ddPCR: 78.7% | p < 0.01 for both | Higher sensitivity; less affected by empiric antibiotics |
| Pneumococcal Carriage in Children (n=374) [70] | 71% | qPCR: 82% | p < 0.001 | Higher detection of pneumococcus and multiple serotypes |
| Necrotizing Soft Tissue Infections (n=20) [16] | 70% | Molecular Methods: 90% | Not Specified | Detection of additional, non-culturable microorganisms |
Table 2: Operational and Economic Comparison of Advanced Sequencing Methods
| Parameter | Metagenomic NGS (mNGS) | Capture-based tNGS | Amplification-based tNGS |
|---|---|---|---|
| Turnaround Time | ~20 hours [71] | Shorter than mNGS [71] | Shortest; suitable for rapid results [71] |
| Cost per Sample | ~$840 [71] | Lower than mNGS [71] | Most cost-effective; for limited resources [71] |
| Pathogen Scope | Broadest (80 species identified) [71] | Targeted, but broad (71 species) [71] | Narrowest (65 species) [71] |
| Best Application | Detection of rare/novel pathogens [71] | Routine diagnostic testing [71] | Rapid results with limited resources [71] |
| Sensitivity (vs. Clinical Diagnosis) | Lower than tNGS [71] | Highest (99.43%) [71] | Poor for some bacteria (e.g., 40.23% for gram-positive) [71] |
Detailed Experimental Protocols and Workflows
Protocol for Multiplex qPCR in Pneumococcal Carriage Studies
The superior performance of qPCR for detecting multiple pneumococcal serotypes, as summarized in Table 1, relies on a precise experimental protocol [70].
- Sample Preparation: Nasopharyngeal swabs are stored in a suitable transport medium and archived frozen. DNA is then extracted from these swabs using standard commercial kits.
- qPCR Assay Design: A series of multiplex qPCR reactions are designed. Each reaction uses primer and probe pairs that are specific to the lytA gene (for universal pneumococcal detection) and to unique capsular polysaccharide biosynthesis genes that define individual serotypes or serogroups.
- Validation and Quantification: The assays are validated for sensitivity, specificity, and linearity. The limit of detection (LOD) for most assays is typically 10 copies per PCR reaction. The quantitative nature of qPCR allows for the estimation of colonization density (in CFU/ml equivalents) and the determination of the dominant and minor serotypes in cases of co-colonization.
- Data Analysis: A sample is considered positive for pneumococcus if the lytA Ct value is below a predetermined threshold. Serotype assignment is made based on positive signals from the specific serotype assays.
Protocol for Metagenomic Next-Generation Sequencing (mNGS)
The mNGS protocol for respiratory and cerebrospinal fluid (CSF) samples demonstrates the comprehensive, culture-independent approach to pathogen diagnosis [72] [64] [71].
- Sample Processing and Host Depletion: A 1 mL sample of BALF or CSF is centrifuged to pellet microbial cells. The pellet is treated with enzymes like Benzonase and detergents like Tween-20 to digest human (host) nucleic acids, thereby enriching the relative proportion of microbial genetic material.
- Nucleic Acid Extraction and Library Construction: Total DNA and RNA are co-extracted. RNA is reverse-transcribed into cDNA. Sequencing libraries are prepared from the combined DNA and cDNA through fragmentation and the addition of platform-specific adapters.
- High-Throughput Sequencing: The library is sequenced on a platform such as the Illumina NextSeq, generating millions of short reads.
- Bioinformatic Analysis: Raw sequencing reads are processed through a rigorous bioinformatic pipeline:
- Quality Filtering: Removal of low-quality reads and adapter sequences.
- Host Read Subtraction: Alignment to a human reference genome (e.g., hg38) to remove remaining host sequences.
- Microbial Identification: Alignment of non-host reads to comprehensive genomic databases containing bacteria, viruses, fungi, and parasites. Pathogens are identified based on the number of unique reads mapped to a specific organism, often normalized as Reads Per Million (RPM). A positive call is made when the RPM in the sample significantly exceeds that in negative control samples.
Visualization of Diagnostic Pathways and Workflows
The following diagrams, generated using Graphviz DOT language, illustrate the logical flow and key decision points in the traditional versus molecular diagnostic pathways.
Diagnostic Pathway Comparison
NGS Method Selection Guide
The Scientist's Toolkit: Essential Research Reagents and Materials
The successful implementation of molecular pathogen discovery requires a suite of specialized reagents and tools. The following table details key components of this toolkit.
Table 3: Essential Reagents and Materials for Molecular Pathogen Diagnostics
| Reagent / Material | Function / Application | Specific Examples |
|---|---|---|
| Nucleic Acid Extraction Kits | Purification of DNA and/or RNA from clinical samples; critical for removing PCR inhibitors. | QIAamp UCP Pathogen DNA Kit [71] / MagPure Pathogen DNA/RNA Kit [71] |
| Host Depletion Reagents | Selective degradation of human nucleic acids to increase the relative abundance of microbial sequences in mNGS. | Benzonase + Tween-20 [64] [71] |
| Reverse Transcriptase & Kits | Conversion of RNA to cDNA for the detection of RNA viruses. | Ovation RNA-Seq system [71] |
| Library Preparation Kits | Preparation of DNA/cDNA fragments for sequencing by adding platform-specific adapters and barcodes. | Ovation Ultralow System V2 [71] |
| Target Enrichment Panels | For tNGS; sets of primers or probes to amplify/capture sequences from a predefined set of pathogens. | Respiratory Pathogen Detection Kit (198 primers) [71] |
| Bioinformatic Databases | Curated genomic reference databases for classifying sequencing reads to specific pathogens. | Self-building clinical pathogen database; Genbank/Refseq [71] |
The evidence clearly demonstrates that molecular methods offer significant advantages over traditional culture in terms of speed, sensitivity, and the ability to detect complex polymicrobial or fastidious infections [16] [70] [64]. However, the choice of method is not a simple binary. The decision matrix reveals a landscape of trade-offs: mNGS offers unparalleled breadth for investigating rare pathogens, while targeted NGS and ddPCR provide more rapid, cost-effective solutions for routine or resource-constrained settings [64] [71]. The most significant barrier to the global adoption of these advanced techniques remains their high cost and infrastructural demands, including stable electricity, sophisticated equipment, and bioinformatic expertise. Future efforts in the field of pathogen discovery must focus not only on technological innovation but also on developing simplified, cost-effective, and robust protocols that can bridge the access gap, ensuring that the benefits of molecular diagnostics are available to researchers and patients in all resource settings.
Clinical microbiology is experiencing a transformative shift from traditional culture-based methods toward sophisticated molecular techniques for pathogen discovery. For decades, culture has served as the cornerstone of clinical microbiology, providing the gold standard for pathogen identification through its ability to isolate viable organisms for further characterization [16]. However, the evolutionary pace of clinical microbiology has accelerated dramatically with the integration of molecular diagnostics, creating a new diagnostic learning curve that is reshaping the profession [73]. This paradigm shift reflects the growing recognition that comprehensive pathogen analysis requires a synergistic approach that leverages the complementary strengths of both methodological families.
The limitations of traditional methods become particularly evident when facing polymicrobial infections, slow-growing pathogens, and cases where previous antimicrobial therapy has compromised culturability [16] [17]. Molecular methods have emerged as powerful alternatives that can detect genetic material from pathogens regardless of their viability or growth requirements, offering unprecedented sensitivity and dramatically reduced turnaround times [74]. Yet, molecular approaches have their own limitations, including the inability to determine pathogen viability and limited utility for antimicrobial susceptibility testing without specific genetic markers [16]. This comparative guide objectively examines the performance characteristics of both approaches and provides a framework for their integration into optimized diagnostic workflows.
Fundamental Methodological Comparisons
Core Principles and Technical Foundations
Culture Methods rely on the propagation of microorganisms in artificial media that support growth, followed by phenotypic identification based on morphological, biochemical, and metabolic characteristics. The fundamental requirement is pathogen viability, with typical incubation periods ranging from 24-48 hours for common bacteria to several weeks for slow-growing organisms like Mycobacterium tuberculosis [16]. Culture remains indispensable for antimicrobial susceptibility testing (AST), which determines phenotypic resistance patterns through disk diffusion, gradient diffusion, or broth microdilution methods [75].
Molecular Methods detect pathogen-specific genetic sequences through various amplification and detection technologies. These include polymerase chain reaction (PCR), which amplifies target DNA sequences through thermal cycling; next-generation sequencing (NGS), which enables comprehensive genomic analysis; and emerging technologies like CRISPR-based systems that allow precise detection of specific DNA/RNA sequences [74]. Molecular methods target conserved genetic regions (e.g., 16S rRNA for bacteria) or specific virulence and resistance genes, providing results within hours rather than days [74].
Performance Comparison: Sensitivity, Specificity, and Turnaround Time
Table 1: Comparative Performance of Culture versus Molecular Methods
| Performance Parameter | Culture Methods | Molecular Methods |
|---|---|---|
| Overall Sensitivity | Limited for fastidious/uncultivable pathogens | Significantly higher, detects non-viable organisms |
| Specificity | High for identifiable organisms | High, dependent on primer/probe design |
| Typical Turnaround Time | 24-72 hours (up to weeks for slow-growers) | 1-6 hours for PCR, 24-72 hours for NGS |
| Polymicrobial Infection Detection | Limited, often misses minority populations | Comprehensive, identifies mixed communities |
| Impact of Prior Antibiotics | Significant reduction in sensitivity | Minimal impact |
| Quantification Capability | Semi-quantitative (CFU/ml) | Quantitative with qPCR/ddPCR |
| Automation Potential | Moderate with modern systems | High for extraction and amplification |
The superior sensitivity of molecular methods is particularly evident in complex infections. A study comparing culture and molecular identification in chronic wounds found that culture identified only 17 different bacterial taxa across 168 wounds, while molecular testing revealed 338 different taxa—nearly 20 times more diversity [17]. Similarly, in necrotizing soft tissue infections, molecular methods identified microorganisms in 90% of surgical samples compared to 70% for culture, frequently detecting additional microorganisms that culture missed [16].
Turnaround time represents another critical differentiator. Traditional blood culture workflows typically require 95-117 hours from collection to final report, while automated molecular workflows can reduce this timeframe to 47-82 hours—a reduction of nearly 37% [75]. This acceleration enables earlier initiation of targeted therapy, which directly improves clinical outcomes.
Experimental Data and Comparative Studies
Direct Method Comparison in Clinical Specimens
Table 2: Summary of Key Comparative Studies
| Study Focus | Sample Type | Culture Results | Molecular Results | Clinical Implications |
|---|---|---|---|---|
| Necrotizing Soft Tissue Infections [16] | Tissue debridement (n=20) | 70% of samples yielded positive culture | 90% of samples positive; detected additional pathogens including fungi, mycoplasma | Altered antimicrobial therapy in polymicrobial cases |
| Chronic Wounds [17] | Wound debridement (n=168) | 17 total bacterial taxa identified | 338 total bacterial taxa identified | Revealed complex biofilm communities impacting treatment |
| Sepsis Diagnostics [76] | Whole blood (n=120) | Reference standard but time-consuming | 77.5% accuracy with optimized DNA extraction | Faster pathogen identification crucial for sepsis outcomes |
| Automated Blood Culture [75] | Blood cultures (pre:192, post:156) | 95.99h baseline TAT | 60.81h TAT with automation | Shorter hospital stays, faster optimal therapy |
The comparative study on necrotizing soft tissue infections utilized multiple molecular methodologies including 16S rRNA gene clone libraries, Ibis T5000 biosensor, and 454 pyrosequencing alongside standard culture [16]. This comprehensive approach demonstrated that half of the patients were infected with Streptococcus pyogenes, but also revealed atypical findings including Acinetobacter baumannii, Streptococcus pneumoniae, and mixed infections involving fungi, mycoplasma, and Fusobacterium necrophorum [16]. The researchers concluded that "no specific 'NSTI causing' combination of species exists," highlighting the importance of broad-spectrum detection capabilities for optimal patient management.
In chronic wound diagnostics, the dramatic discrepancy between culture and molecular identification has profound implications for understanding wound bioburden. The biofilm phenotype predominant in chronic wounds creates intrinsic resistance to both antimicrobial agents and detection by conventional culture [17]. Molecular methods revealed complex polymicrobial communities that interact synergistically, potentially explaining why chronic wounds often fail to respond to therapy targeted at a single pathogen isolated by culture.
DNA Extraction Methodologies: A Critical Pre-Analytical Factor
The efficiency of molecular diagnostics depends significantly on pre-analytical processes, particularly DNA extraction. A 2025 study compared three DNA extraction methods for sepsis-causing pathogens in whole blood: one column-based method and two magnetic bead-based methods [76]. The results demonstrated superior performance with magnetic bead-based technologies, which achieved 77.5% accuracy for Escherichia coli detection compared to 65.0% with the column-based method [76]. For Staphylococcus aureus detection, the automated GraBon system achieved 77.5% accuracy versus 67.5% with other methods [76]. These findings highlight how methodological choices in DNA extraction significantly impact downstream diagnostic accuracy.
Integrated Workflow Design
Complementary Workflow Strategy
The most effective diagnostic approach leverages both culture and molecular methods in a complementary manner. Molecular methods provide rapid screening and comprehensive pathogen detection, while culture maintains its essential role in antimicrobial susceptibility testing and isolating viable organisms for further investigation. This integrated model maximizes diagnostic accuracy while preserving crucial functionality for treatment guidance.
Laboratory Implementation and Workflow Optimization
Implementing integrated workflows requires careful laboratory design to prevent contamination, particularly in molecular diagnostics. A unidirectional workflow is essential, moving from clean pre-amplification areas to post-amplification zones with strict physical separation [77]. Laboratory layout should include:
- Reagent Preparation Room: A dedicated clean space for preparing amplification master mixes
- Sample Preparation Room: Separate area for nucleic acid extraction
- Amplification Room: Designated space for thermal cycling and post-PCR analysis [77]
This physical separation prevents amplicon contamination that can compromise test results. Laboratories with space constraints can implement dead air boxes or biological safety cabinets to create contained workstations for specific procedures [77].
Automation represents another critical optimization strategy. Integrated automated systems for blood culture diagnostics have demonstrated significant reductions in turnaround time—from 95.99 hours to 60.81 hours—without additional staffing [75]. These systems combine automated loading/unloading of blood culture bottles, automatic subculturing of positive cultures, expert system-based review of AST results, and automated reporting of negative results [75].
Essential Research Reagent Solutions
Successful implementation of integrated pathogen discovery workflows requires specific reagent systems and laboratory materials. The following table summarizes essential solutions and their applications in culture and molecular methodologies.
Table 3: Essential Research Reagent Solutions for Integrated Workflows
| Reagent/Material | Application | Function | Method Category |
|---|---|---|---|
| QIAamp DNA Blood Mini Kit [76] | DNA extraction from whole blood | Column-based nucleic acid purification | Molecular |
| K-SL DNA Extraction Kit [76] | Bacterial DNA extraction | Magnetic bead-based purification with bacterial isolation | Molecular |
| GraBon Automated System [76] | High-throughput DNA extraction | Automated magnetic bead-based platform | Molecular |
| BacT/Alert Virtuo BC System [75] | Blood culture incubation | Automated continuous monitoring of microbial growth | Culture |
| Vitek 2 XL Microbiology Analyzer [75] | Identification & AST | Automated phenotypic identification and susceptibility testing | Culture |
| HotStarTaq Plus Master Mix [17] | 16S rRNA amplification | PCR amplification for microbial community analysis | Molecular |
| Gibco Cell Culture Media [78] | Mammalian cell culture | Support growth of cell lines for host-pathogen studies | Culture |
| VPlus 50 Automated Sample Processor [75] | Sample preparation | Automated subculturing of positive blood cultures | Culture/Molecular |
The integration of culture and molecular methods represents the future of comprehensive pathogen analysis in clinical and research settings. Rather than positioning these methodologies as competitors, the optimal approach leverages their complementary strengths—harnessing the speed and sensitivity of molecular techniques for rapid pathogen detection while maintaining the phenotypic characterization capabilities of culture for antimicrobial guidance. This synergistic model maximizes diagnostic accuracy while providing clinically actionable information in timeframes that meaningfully impact patient management.
As molecular technologies continue to evolve—with advances in point-of-care testing, portable sequencing, and automated platforms—the integration of these methodologies will become increasingly seamless. The future of pathogen discovery lies not in choosing between methodological approaches, but in developing unified workflows that intelligently combine their capabilities to provide a more complete understanding of infectious diseases. This integrated paradigm promises to accelerate diagnostic timelines, enhance antimicrobial stewardship, and ultimately improve patient outcomes across the spectrum of infectious diseases.
Head-to-Head: Validating Performance of Molecular vs. Culture Methods
The field of pathogen discovery is undergoing a fundamental transformation, moving from traditional culture-based methods to sophisticated molecular techniques. This shift is driven by a "comparison culture" within the scientific community—a rigorous, data-driven ethos that demands quantitative evidence of diagnostic superiority. This comparative guide synthesizes current experimental data to objectively evaluate the performance of established and emerging molecular diagnostics against conventional methods across clinical applications. The metrics are clear: molecular methods consistently demonstrate profound advantages in detection rates and turnaround times, enabling more precise and timely clinical interventions [79] [80] [81].
The following analysis provides researchers, scientists, and drug development professionals with a detailed, evidence-based resource for selecting appropriate diagnostic tools. We present summarized quantitative data, detailed experimental methodologies, and visualizations of key workflows to illuminate the technical foundations of this diagnostic revolution.
Comparative Performance Data Across Applications
The tables below synthesize performance data from recent studies, providing a direct comparison between traditional and molecular diagnostic methods.
Table 1: Detection Rate and Turnaround Time Comparisons
| Clinical Application | Method | Detection Rate | Turnaround Time | Key Performance Metrics | Source |
|---|---|---|---|---|---|
| Pneumonia Diagnosis | Traditional Culture | 61.6% (Spring), 56.8% (Winter) | 48-50 hours (median) | Reference standard | [79] |
| Seasonal PCR Panel | 80.6% (Spring), 80.0% (Winter) | 12-14 hours (median) | Risk Difference: +19.0 pp (Spring), +22.3 pp (Winter); p<0.01 | [79] | |
| Neurosurgical CNS Infections | Microbial Culture | 59.1% | 22.6 ± 9.4 hours (Mean THTR*) | Reference standard | [80] |
| Metagenomic NGS (mNGS) | 86.6% (p<0.01) | 16.8 ± 2.4 hours (Mean THTR*) | 29.1% of patients positive only by mNGS | [80] | |
| Droplet Digital PCR (ddPCR) | 78.7% (p<0.01) | 12.4 ± 3.8 hours (Mean THTR*) | Significantly shorter THTR vs. mNGS (p<0.01) | [80] | |
| MRSA Detection | Culture & Susceptibility | Reference | 48-72 hours | Reference standard | [81] |
| CRISPR-Based Methods | 99% Pooled Sensitivity (97-100% CI), 100% Specificity (99-100% CI) | 60 minutes (median, IQR: 41-99 min) | Diagnostic Odds Ratio: 664.25; PLR: 32.68 | [81] |
*THTR: Time from Sample Harvesting to Final Positive Result
Table 2: Impact on Clinical Decision-Making and Workflow
| Clinical Application | Method | Impact on Antibiotic Stewardship / Clinical Workflow | Other Significant Outcomes | Source |
|---|---|---|---|---|
| Pneumonia Diagnosis (Winter Cohort) | Traditional Culture | Guideline-concordant empiric therapy: 64.9% | Antibiotic changes ≤72h: 28.4% | [79] |
| Seasonal PCR Panel | Guideline-concordant empiric therapy: 78.7% (+13.8 pp) | Antibiotic changes ≤72h: 14.7% (-13.7 pp); Mean antibiotic courses shortened by 1.5-1.7 days | [79] | |
| Comprehensive Genomic & Immune Profiling (Cancer) | Multi-modal CGIP (NGS+IHC) | 61.0% of cases had ≥1 Tier 1 biomarker; 88.8% had ≥1 Tier 2 biomarker | Median turnaround: 8 days; Test success: 96.0% (DNA NGS) to 99.8% (PD-L1 IHC) | [82] |
Detailed Experimental Protocols and Methodologies
Protocol 1: Evaluation of Season-Specific PCR Panels for Pneumonia
Objective: To evaluate whether season-tailored multiplex PCR panels accelerate pathogen identification and improve antibiotic stewardship compared to conventional diagnostics in emergency department patients with pneumonia [79].
Study Design:
- A prospective, single-center, quasi-randomized comparative study.
- Participants were allocated via a rotating week-on/week-off schedule to either a seasonal PCR panel or conventional diagnostics.
- The trial period ran from January 2022 to October 2024, encompassing predefined respiratory-infection seasons (Spring: March-May; Autumn-Winter: October-February).
Participant Eligibility:
- Inclusion Criteria: Adults (≥18 years) with new respiratory symptoms (≤10 days) and imaging-confirmed pulmonary infiltrate.
- Exclusion Criteria: Prolonged antibiotic exposure (>72 hours) before ED presentation, severely immunocompromised state, non-infectious infiltrates, or inability to provide consent.
PCR Panel Development:
- Two distinct seasonal PCR panels were designed based on local surveillance data from the Shanghai Municipal CDC.
- The Spring Panel targeted Influenza A/B, Parainfluenza, Rhinovirus/Enterovirus, Adenovirus, S. pneumoniae, H. influenzae, M. pneumoniae, C. pneumoniae, and L. pneumophila.
- The Autumn-Winter Panel targeted Influenza A/B, Human Metapneumovirus, Rhinovirus/Enterovirus, seasonal Coronaviruses (non-SARS-CoV-2), RSV A/B, S. pneumoniae, H. influenzae, S. aureus, M. pneumoniae, C. pneumoniae, L. pneumophila, and K. pneumoniae.
- SARS-CoV-2 was excluded as mandatory standalone testing was already in place.
Outcome Measures:
- Primary: Time to final pathogen report and diagnostic yield (≥1 pathogen detected).
- Secondary: Empiric-antibiotic appropriateness within 24h, regimen changes ≤72h, antibiotic duration, length of stay, and 30-day mortality.
Statistical Analysis:
- Power analysis assumed a 15-20% improvement in detection rates or a 12-24-hour reduction in turnaround time.
- Target enrollment was approximately 75 patients per arm per season to detect clinically meaningful differences (α: 0.05, power: 80%) [79].
Protocol 2: Diagnostic Evaluation of mNGS and ddPCR for Neurosurgical CNS Infections
Objective: To comprehensively assess the diagnostic performance of metagenomic next-generation sequencing (mNGS) and Multiplex Droplet Digital PCR (ddPCR) in elucidating the microbiological etiologies of neurosurgical central nervous system infections (NCNSIs) [80].
Study Design and Population:
- A cohort of 127 patients with clinically diagnosed NCNSIs was enrolled from the Emergency Neurosurgical Intensive Care Unit.
- Study period: June 2022 to October 2024.
- Inclusion Criteria: Based on clinical symptoms (headache, fever ≥38.5°C, meningeal irritation), CSF findings (WBC ≥1000/μL, polykaryocyte percentage ≥75%, glucose <2.5 mmol/L or CSF/blood glucose ratio <0.4), and imaging results (dural enhancement, ventricular dilation, or ring-enhancing lesions).
Sample Processing and Testing:
- Cerebrospinal fluid (CSF) samples were harvested via lumbar puncture or drainage tubes. Abscess samples were obtained during surgery.
- All samples underwent parallel testing via:
- Traditional Culture: Time to positive culture (TTPC) and time from sample harvesting to result (THTR) were recorded.
- mNGS: Conducted for unbiased, high-throughput pathogen detection.
- ddPCR: Performed for highly sensitive and quantitative molecular detection.
Data Collection and Analysis:
- Data collected included clinical records, laboratory results, TTPC, THTR for all methods, turnaround times, and 3-month follow-up data.
- Pathogen detection rates were compared using statistical tests (p<0.01 considered significant).
- THTR was compared between methods, with a significant difference noted between ddPCR and mNGS (p<0.01) [80].
Visualizing Workflows and Signaling Pathways
Seasonal PCR Panel Implementation Workflow
CRISPR-Cas System for MRSA Detection
The Scientist's Toolkit: Essential Research Reagents and Solutions
Table 3: Key Research Reagent Solutions for Molecular Pathogen Detection
| Item | Function in Research/Diagnostics | Specific Application Example |
|---|---|---|
| Multiplex PCR Panels | Simultaneously detects multiple pathogens from a single sample, maximizing data yield from limited specimen. | BioFire FilmArray Pneumonia Panel [83] |
| CRISPR-Cas Reagents | Provides sequence-specific recognition and cleavage of nucleic acids, enabling highly specific pathogen detection. | MRSA detection targeting mecA gene [81] |
| Next-Generation Sequencing Kits | Allows for unbiased, hypothesis-free detection of pathogens through high-throughput sequencing. | TruSight Oncology 500 for comprehensive genomic profiling [82] |
| Nucleic Acid Extraction Kits | Isolates high-quality DNA and RNA from clinical specimens (e.g., FFPE, CSF), crucial for downstream analysis. | truXTRAC FFPE extraction kit for co-extraction of DNA/RNA [82] |
| Automated Molecular Platforms | Streamlines testing procedures, reduces technician hands-on time, and minimizes operational variability. | Integrated systems for PCR panel processing [84] |
| Specialized Culture Media | Supports the growth of fastidious microorganisms, serving as a reference standard for method comparison. | Culture on blood, chocolate, and MacConkey agars [79] |
The quantitative data presented in this guide leaves little doubt about the diagnostic superiority of molecular methods over traditional culture-based techniques. The evidence consistently shows significant improvements in detection rates—often by 20-30 percentage points—and dramatic reductions in turnaround time—from days to hours. These advancements are not merely incremental; they represent a fundamental shift in diagnostic capability that directly impacts patient care through improved antibiotic stewardship, more targeted therapies, and streamlined clinical workflows [79] [80] [81].
This comparative analysis, grounded in robust experimental protocols and current data, provides a definitive resource for the scientific community. It validates the transition to molecular diagnostics as not just a technological upgrade, but a necessary evolution to meet the demands of modern precision medicine. As molecular techniques continue to evolve, this "comparison culture" will remain essential for rigorously validating new methodologies and ensuring that diagnostic science continues to advance in step with therapeutic innovation.
In the high-stakes realm of clinical microbiology, time is a critical determinant of patient outcomes. Turnaround time (TAT) and time to positivity (TTP) represent two fundamental metrics that gauge the speed and efficiency of pathogen detection, directly influencing therapeutic decisions and survival rates. Turnaround time encompasses the total duration from test ordering to result reporting, reflecting the overall laboratory service efficiency [85]. Time to positivity, more specific to microbiology, measures the interval from the start of blood culture incubation to the automated detection of microbial growth [86] [87] [88]. As the diagnostic landscape evolves with emerging molecular technologies, a persistent tension exists between traditional culture-based methods—long considered the gold standard—and rapid molecular techniques. This analysis objectively compares the performance of these methodological approaches through the lens of TAT and TTP, providing researchers and drug development professionals with evidence-based insights to guide diagnostic strategies and antimicrobial development.
Defining the Metrics: TAT and TTP in Practice
Turnaround Time (TAT): The Total Testing Cycle
Laboratory TAT represents the total testing cycle, which can be classified into pre-analytical (test ordering to sample preparation), analytical (actual test performance), and post-analytical (result reporting to clinical action) phases [85]. Clinicians and laboratories often differ in their definitions of TAT; over 40% of physicians define TAT as starting at physician request, while only 9% use laboratory receipt as the start point [85]. This discrepancy highlights the importance of standardizing metrics when comparing diagnostic approaches. Due to its non-Gaussian distribution with positive skew, TAT is best measured using median and tail size metrics (e.g., 90th percentile completion time) rather than means and standard deviations [85].
Time to Positivity (TTP): A Measure of Microbial Growth Dynamics
TTP serves as both a diagnostic and prognostic tool in bloodstream infections, reflecting the initial bacterial load at collection time—shorter TTP generally indicates higher bacterial burden [88]. The clinical interpretation of TTP is multifaceted: shorter TTP values correlate with higher microbial loads, more severe infections, and increased mortality rates [86] [87] [88]. Technological advances have necessitated recalibration of TTP thresholds; for instance, a 2025 study established 20 hours as the optimal cutoff for differentiating true bacteremia from contamination with major contaminants like coagulase-negative staphylococci, revising the traditional 24-hour benchmark [89].
Comparative Performance: Culture vs. Molecular Methods
Detection Rates and Speed
Molecular techniques demonstrate superior detection rates and speed compared to traditional culture methods across multiple infection types and sample sources.
Table 1: Detection Rate Comparison Between Methods
| Infection Type | Sample Source | Culture Positive Rate | mNGS Positive Rate | ddPCR Positive Rate | Study |
|---|---|---|---|---|---|
| Neurosurgical CNS Infections | Cerebrospinal fluid/Pus | 59.1% | 86.6% (p<0.01) | 78.7% (p<0.01) | [80] |
| Kidney Transplantation | Organ Preservation Fluids | 24.8% | 47.5% (p<0.05) | N/A | [90] |
| Kidney Transplantation | Recipient Wound Drainage Fluids | 2.1% | 27.0% (p<0.05) | N/A | [90] |
| Necrotizing Soft Tissue Infections | Tissue Samples | 70% | 90% (multiple molecular methods) | N/A | [16] |
Table 2: Time Metric Comparison Between Methods
| Method | Time to Positive Result (Hours) | Total Time to Final Result (THTR) | Notes | Study |
|---|---|---|---|---|
| Microbial Culture | 15.1 ± 10.4 (Mean TTP) | 22.6 ± 9.4 hours | For neurosurgical CNS infections | [80] |
| mNGS | N/A | 16.8 ± 2.4 hours | Significantly longer than ddPCR (p<0.01) | [80] |
| ddPCR | N/A | 12.4 ± 3.8 hours | Significantly shorter than mNGS (p<0.01) | [80] |
| Enhanced TTP Assay | Variable | N/A | Includes bottles with antimicrobial agents | [86] |
Methodological Advantages and Limitations
Each diagnostic approach offers distinct advantages and suffers from specific limitations:
Traditional Culture Methods maintain the irreplaceable advantage of allowing subsequent antibiotic susceptibility testing, antigenic studies, experimental models, and genetic studies [66]. Culture remains the ultimate goal of pathogen identification but is hampered by lengthy processing times (typically 15-28 hours for TTP alone) [80] [66]. Culture sensitivity is significantly compromised by prior antibiotic administration [16], and standard TTP interpretation is often empirical, potentially confounding therapy design [86].
Molecular Methods including mNGS and ddPCR offer rapid, culture-independent detection with superior sensitivity [80] [90]. mNGS provides unbiased, high-throughput pathogen detection capable of identifying novel, rare, and fastidious organisms without prior hypothesis about potential pathogens [80]. ddPCR offers exceptional sensitivity, reproducibility, simplicity, and speed for targeted detection [80]. However, molecular methods may fail to detect some organisms identified by culture (e.g., only 22.2% of culture-detected Gram-positive bacteria and 55.6% of fungi were detected by mNGS in one transplant study) [90], and cannot provide antibiotic susceptibility profiles.
Advanced Applications and Interpretative Frameworks
Enhanced TTP Paradigms
Innovative approaches to TTP utilization are emerging that enhance its clinical utility. The enhanced TTP assay augments standard diagnostic TTP by incorporating blood culture bottles containing antimicrobial agents at various concentrations (alone or in combination) alongside standard culture bottles [86]. When coupled with mathematical modeling, this enhanced approach enables better-informed guidance for antimicrobial therapy by simultaneously assessing microbial population growth and bactericidal effects of antimicrobial agents [86]. This methodology addresses the ambiguity of empirical TTP interpretation, where identical TTP values may originate from infections with different growth rates or initial microbial loads [86].
Prognostic and Diagnostic Applications
TTP provides valuable clinical insights beyond mere pathogen detection:
- Catheter-Related Infection Identification: For Pseudomonas aeruginosa bloodstream infections, a TTP <13 hours combined with differential TTP (DTP) >2 hours independently predicted catheter-related sources [87].
- Mortality Risk Stratification: Shorter TTP significantly correlates with higher 30-day mortality. For catheter-related P. aeruginosa infections, TTP <14 hours exacerbated mortality among patients without catheter removal within 48h (OR 2.9), while for other sources, TTP <16h increased mortality (OR 1.6) [87].
- Contamination Differentiation: A 20-hour TTP threshold effectively differentiates true bacteremia from contamination with major contaminants like coagulase-negative staphylococci and viridans group streptococci [89].
Essential Research Reagent Solutions
Table 3: Key Research Reagents and Platforms for TAT/TTP Studies
| Reagent/Platform | Function | Application Context |
|---|---|---|
| BACTEC FX/FX40 (Becton Dickinson) | Automated blood culture incubation and monitoring | Standard TTP determination [90] |
| BACT/ALERT VIRTUO (bioMérieux) | Automated blood culture system with colorimetric detection | TTP studies in population cohorts [88] |
| BD BACTEC Plus Aerobic/F Culture Vials | Resin-containing media for blood culture | Conventional culture comparisons [90] |
| MALDI-TOF MS (Bruker Daltonics) | Rapid microbial identification from positive cultures | Post-TTP pathogen identification [90] |
| PACEseq mNGS (Hugobiotech) | Metagenomic next-generation sequencing workflow | Culture-independent pathogen detection [90] |
| QIAamp DNA Micro Kit (QIAGEN) | Cell-free DNA extraction from clinical samples | mNGS sample preparation [90] |
The comparative analysis of TAT and TTP metrics reveals a nuanced diagnostic landscape where neither culture nor molecular methods universally prevail. Instead, their complementary strengths suggest an integrated approach maximizes diagnostic efficacy. Traditional culture methods remain indispensable for antibiotic susceptibility testing and comprehensive pathogen characterization but suffer from longer turnaround times and lower sensitivity in antibiotic-exposed patients. Molecular techniques (mNGS and ddPCR) provide rapid, sensitive detection with significantly shorter TAT, enabling earlier targeted therapy, but cannot determine antibiotic susceptibility and may miss some culture-detectable organisms.
For researchers and drug development professionals, these findings highlight several critical considerations: First, the choice between methods should be guided by clinical context, urgency, and information needs rather than hierarchical preference. Second, ongoing technological advances continue to recalibrate performance metrics, as evidenced by the revised 20-hour TTP cutoff for contamination differentiation. Finally, innovative approaches like enhanced TTP assays with mathematical modeling represent promising frontiers for optimizing antimicrobial therapy guidance. As diagnostic technologies evolve, continuous re-evaluation of these fundamental timing metrics will remain essential for advancing patient care and therapeutic development.
The accurate and timely identification of pathogens is a cornerstone of effective infectious disease management. For decades, conventional culture-based methods have served as the gold standard in clinical microbiology. However, their performance is significantly compromised in complex clinical scenarios, particularly in polymicrobial infections (PMIs) and cases involving prior antibiotic exposure. These challenges have catalyzed a paradigm shift in pathogen discovery research, fueling the development and adoption of molecular diagnostic techniques. This guide provides a comparative analysis of the performance of classical culture methods against advanced molecular techniques, focusing on their ability to detect pathogens that traditionally evade diagnosis. Framed within a broader thesis on diagnostic evolution, we present experimental data and methodologies that underscore the transformative potential of molecular platforms in modern microbiology.
Comparative Performance of Diagnostic Methods
The limitations of conventional culture are most apparent in its suboptimal sensitivity and lengthy turnaround time, which can critically delay targeted therapy. The tables below summarize the comparative performance of different diagnostic approaches as revealed by recent studies.
Table 1: Overall Comparative Performance of Culture vs. Molecular Methods
| Method | Sensitivity in PMIs | Time to Result | Key Advantages | Key Limitations |
|---|---|---|---|---|
| Conventional Culture | Detects only 60-70% of co-pathogens in PMIs [91] | 24-72 hours [92] [93] | Low cost, provides isolate for AST [94] | Misses uncultivable, slow-growing, and pre-exposed pathogens [91] |
| Multiplex PCR | Limited by predefined targets [92] | 1-4 hours [92] | Rapid, high-throughput | Fixed panel; cannot detect novel or unexpected pathogens [92] |
| Metagenomic Next-Generation Sequencing (mNGS) | >95% for polymicrobial identification; detects additional 8.5% of infections [92] [95] | ~3.5 hours from positive blood culture [92] | Unbiased, comprehensive pathogen detection [92] [91] | Higher cost, complex data analysis, requires specialized reagents [91] |
Table 2: Quantitative Performance Data from Key Studies
| Study (Method) | Sample Type | Key Performance Metric vs. Culture | Result |
|---|---|---|---|
| Govender & Eyre 2025 (mNGS) [92] | 211 Positive Blood Cultures | Sensitivity (Species ID) | 97% (improving to 100% with optimized thresholds) |
| Specificity (Species ID) | 94% | ||
| Additional Infections Detected | 18 (13 polymicrobial, 5 previously unidentifiable) | ||
| Time to Result | 3.5 hours ( |
||
| Frontiers in Cell & Infect. Micro. 2025 (mNGS) [95] | Organ Preservation & Wound Fluids | Positive Rate in Preservation Fluids | mNGS: 47.5% vs. Culture: 24.8% (p<0.05) |
| Positive Rate in Wound Fluids | mNGS: 27.0% vs. Culture: 2.1% (p<0.05) | ||
| Scientific Reports 2025 (Automated DNA Extraction) [76] | Clinical Whole Blood | Accuracy for E. coli Detection (Magnetic Bead-based vs. Column-based) | 77.5% vs. 65.0% (p<0.05) |
Detailed Experimental Protocols
To ensure reproducibility and provide a clear understanding of the generating evidence, this section details the key methodologies from the cited studies.
This protocol describes the optimized pipeline for rapid pathogen identification and antimicrobial resistance (AMR) prediction directly from positive blood culture bottles.
- Sample Preparation: A total of 273 randomly selected blood culture bottles (211 positive, 62 negative) were processed. Nucleic acids were extracted directly from the blood culture broth, bypassing the need for subculture.
- Library Preparation & Sequencing: DNA libraries were prepared for sequencing on the Oxford Nanopore Technologies (ONT) platform, chosen for its rapid turnaround time.
- Bioinformatic Analysis: Sequencing reads were analyzed using a customized workflow. For species identification, three methods were tested using Bracken with a standard database, adjusted for contamination.
- Method 1: Standard analysis excluding plasmid sequences.
- Method 2: Added genome mapping with heuristic thresholds (number of species reads and percentage of bacterial reads from the species).
- Method 3: Used mapping with random forest model-derived thresholds.
- Antimicrobial Resistance (AMR) Prediction: The pipeline also included a step for predicting AMR by detecting resistance genes within the metagenomic data, which was compared to phenotypic susceptibility testing results.
This study evaluated the impact of DNA extraction efficiency, a critical pre-analytical step, on the molecular diagnostic accuracy of sepsis-causing pathogens.
- Sample Collection: 120 whole blood samples were used, including 40 with S. aureus, 40 with E. coli, 20 culture-negative, and 20 from healthy checkups.
- Extraction Methods Compared:
- QIAamp DNA Blood Mini Kit (QIAGEN): A manual, column-based method.
- K-SL DNA Extraction Kit (KingoBio): A manual, magnetic bead-based method that includes a bacterial isolation step.
- GraBon System (KingoBio): An automated platform using the same magnetic bead-based chemistry as the K-SL kit.
- Downstream Analysis: The extracted DNA from all three methods was subjected to real-time PCR using specific primers for S. aureus and E. coli. The cycle threshold (Ct) values and detection rates were compared to determine the accuracy of each method.
Visualization of Diagnostic Pathways
The following workflow diagrams illustrate the procedural and logical differences between conventional and modern molecular diagnostic pathways for bloodstream infections.
Conventional Culture-Based Diagnostic Pathway
Molecular Diagnostic Pathway
The Scientist's Toolkit: Key Research Reagent Solutions
The experiments cited rely on specialized reagents and platforms to achieve their performance. The following table details these essential tools and their functions.
Table 3: Essential Research Reagents and Platforms for Advanced Pathogen Detection
| Reagent/Platform | Function in Workflow | Key Feature/Benefit |
|---|---|---|
| Oxford Nanopore Sequencing Platforms [92] | Long-read, real-time sequencing | Enables rapid (~3.5 hour) sequencing direct from complex samples like blood cultures. |
| QIAamp DNA Blood Mini Kit (QIAGEN) [76] | Manual, column-based DNA purification | A widely used benchmark method for nucleic acid extraction from blood. |
| K-SL DNA Extraction Kit (KingoBio) [76] | Manual, magnetic bead-based DNA purification with bacterial isolation | Isolates bacteria from whole blood before lysis, improving purity and yield. |
| GraBon Automated System (KingoBio) [76] | Automated, magnetic bead-based nucleic acid extraction platform | Provides consistency, higher throughput, and efficient lysis via vigorous mechanical disruption. |
| Bracken Software [92] | Bioinformatic tool for species abundance estimation from metagenomic data | Allows for accurate taxonomic classification and quantification in polymicrobial samples. |
The comparative data presented in this guide substantiate a definitive shift in the paradigm of pathogen discovery. Conventional culture, while foundational, exhibits significant diagnostic gaps in the face of polymicrobial and antibiotic-pretreated infections. Molecular methods, particularly clinical metagenomics, demonstrate superior performance by delivering unprecedented sensitivity, a drastically reduced time-to-result, and a comprehensive diagnostic scope that captures the true complexity of these challenging infections [92] [95] [91]. The integration of robust automated extraction systems [76] and advanced bioinformatic pipelines [92] is crucial for realizing the full potential of these technologies. As these molecular platforms continue to evolve and become more accessible, they are poised to become the new cornerstone of clinical microbiology, ultimately guiding more precise and timely therapeutic interventions and improving patient outcomes.
In the field of pathogen discovery and antimicrobial resistance (AMR) surveillance, a fundamental comparison exists between classical culture-based methods and modern molecular techniques. Antimicrobial Susceptibility Testing (AST) stands as a critical function of clinical microbiology laboratories, providing essential data to guide effective antimicrobial therapy and steward the use of antibiotics [96]. Despite the rapid advancement of molecular diagnostics, which offer genotypic resistance profiles in hours, phenotypic culture-based methods retain a vital, enduring niche. They deliver a direct, measurable assessment of bacterial response to antibiotics, unaffected by the vast, often uncharted, complexity of bacterial resistance mechanisms [97]. This guide objectively compares the performance of traditional culture-based AST with emerging molecular and artificial intelligence (AI)-driven alternatives, framing the discussion within the broader thesis of culture versus molecular methods in pathogen research.
The global context makes this comparison urgent. AMR was associated with an estimated 4.95 million deaths in 2019 and is projected to become the leading cause of mortality worldwide by 2050 [96] [98]. This crisis is fueled by the empirical overuse of antibiotics, a practice often necessitated by the slow turnaround time of conventional culture-based AST, which can require 72 hours or more from specimen collection to final results [97]. While molecular and rapid phenotypic technologies promise to close this timing gap, their validation and implementation—particularly against the benchmark of culture-based methods—remain a core challenge for researchers, scientists, and drug development professionals.
Comprehensive Comparison of AST Methodologies
Performance Characteristics of Major AST Platforms
The following table summarizes the core principles, advantages, and limitations of the primary AST methods in use and development, highlighting the critical performance differentiators.
Table 1: Comparative Analysis of Key Antimicrobial Susceptibility Testing (AST) Methodologies
| Method Category | Specific Method | Underlying Principle | Time to Result (after isolation) | Key Advantages | Major Limitations |
|---|---|---|---|---|---|
| Classic Phenotypic (Culture) | Disk Diffusion (Kirby-Bauer) | Measures zone of inhibition around antibiotic disk [98]. | 16-24 hours [96] | Simple operation, low cost, suitable for large-scale screening [98]. | Cannot determine Minimum Inhibitory Concentration (MIC); results influenced by protocol standardization [98]. |
| Broth Dilution | Determines MIC by visually assessing bacterial growth in antibiotic serial dilutions [96] [98]. | 18-24 hours [96] | Provides precise, quantitative MIC data for personalized therapy [98]. | Labor-intensive, requires specialized equipment [98]. | |
| Rapid Phenotypic | Automated AST Systems | Detects bacterial growth via optical/fluorescence signals to calculate MIC [98]. | 6-24 hours [96] [98] | High-throughput, faster results, standardized [96] [98]. | Expensive instrumentation and maintenance; may require specific FDA clearance for breakpoint updates [99] [98]. |
| E-test (Gradient Diffusion) | Uses a stable antibiotic gradient strip to measure MIC directly on agar [98]. | 16-24 hours | Combines simplicity and precision; ideal for fastidious pathogens [98]. | High reagent costs; limited accessibility in resource-poor regions [98]. | |
| Genotypic | Molecular Methods (PCR, WGS) | Identifies known resistance genes or mutations [96] [98]. | 2-6 hours [96] [98] | Rapid, predicts resistance from primary samples or unculturable pathogens [98] [97]. | Cannot detect novel or complex resistance mechanisms; overestimates resistance if gene is not expressed [96] [97]. |
| Emerging Technologies | AI-Driven Prediction | Machine/Deep Learning analyzes imaging and lab data to predict resistance [98]. | Potentially minutes to hours | Potential for extremely rapid results and insights from complex datasets [98]. | Early stage of development; requires extensive validation and large, curated datasets [98]. |
Experimental Data and Performance Metrics
In head-to-head evaluations, the agreement between methods is a critical metric. Automated systems and gradient diffusion tests (E-test) are typically validated against the reference broth microdilution (BMD) method. Essential Agreement (EA), defined as the percentage of MIC results within one doubling dilution of the reference method, and Categorical Agreement (CA), the percentage of identical interpretations (Susceptible, Intermediate, Resistant), are key performance indicators [99]. Major errors (false resistance) and very major errors (false susceptibility) are also calculated, with the latter being particularly critical for patient safety.
For genotypic methods, the performance is measured by sensitivity and specificity for detecting known resistance markers. However, a significant limitation is that a carbapenemase gene is identifiable in fewer than 50% of bacteria found to be phenotypically carbapenem resistant, highlighting a major gap where culture-based phenotyping remains essential [97].
Validation Frameworks and Experimental Protocols
The Critical Role of Breakpoints and Standards
A cornerstone of AST validation is the application of correct clinical breakpoints. Breakpoints are pre-determined MIC values or zone diameter measurements that categorize an organism as Susceptible (S), Intermediate (I), or Resistant (R) to an antibiotic [99]. These breakpoints are established by standards organizations like the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) based on pharmacological and clinical data.
Using outdated breakpoints poses a direct risk to patients. A real-world scenario illustrates this: a patient's isolate was reported as "susceptible" at one hospital using old breakpoints, but their health worsened. At a second hospital, the same isolate was correctly categorized as "resistant" using updated breakpoints, necessitating a life-saving change in therapy [99]. Consequently, the College of American Pathologists (CAP) now requires all clinical laboratories to update their AST breakpoints by January 1, 2024 [99].
Table 2: Key Research Reagent Solutions and Materials for AST Validation
| Reagent/Material | Function in AST Experimentation | Application Context |
|---|---|---|
| Cation-Adjusted Mueller-Hinton Broth (CAMHB) | Standardized growth medium for broth microdilution [96]. | Reference method validation; quality control. |
| Mueller-Hinton Agar Plates | Standardized solid medium for disk diffusion and E-test [96]. | Phenotypic susceptibility profiling. |
| CLSI M100 Document | Compendium of current, validated breakpoints for interpretation [99]. | Mandatory for accurate result reporting and method validation. |
| Quality Control (QC) Strains | Reference strains with known MIC ranges (e.g., E. coli ATCC 25922, S. aureus ATCC 29213) [99]. | Daily verification of test accuracy and reagent performance. |
| Antimicrobial Powder/Disks | Pure chemical or impregnated disks for creating dilution series or diffusion zones [96]. | Core component of all phenotypic AST methods. |
| FDA-Cleared Automated AST Panels | Standardized panels with lyophilized antibiotics for automated systems [99]. | High-throughput clinical testing. |
Protocol for AST Method Validation and Verification
When implementing a new AST method or updating breakpoints, laboratories must perform rigorous validation. The process differs for verified (on-label) and validated (off-label) use on automated systems [99]. The following workflow outlines the key steps for this critical process.
Validation/Verification Experimental Protocol:
- Strain Selection: Test a panel of 50-100 well-characterized bacterial isolates, including QC strains and clinical isolates with known resistance mechanisms [99].
- Comparative Testing: Perform parallel testing with the new method (e.g., automated system with updated breakpoints) and the reference BMD method [99].
- Data Analysis: Calculate Essential Agreement (EA), Categorical Agreement (CA), and error rates (major and very major errors). The acceptable performance is based on CLSI guidelines [99].
- Documentation: Meticulously document all data from the validation process, as this is required for regulatory inspections [99].
Analysis and Future Directions
The Enduring Niche of Culture-Based Validation
Despite the allure of speed, culture-based phenotypic AST maintains its fundamental role as the reference standard for validation for several reasons. It is a hypothesis-free approach, capable of detecting resistance regardless of the underlying mechanism—be it enzymatic inactivation, target alteration, membrane permeability changes, or efflux pump activity [96]. This is in stark contrast to genotypic methods, which can only detect the specific resistance genes they are designed to probe. Since a large proportion of phenotypic resistance, particularly in Gram-negative bacteria, arises from complex, multifactorial, or novel mechanisms not covered by standard gene panels, culture remains indispensable [97].
Furthermore, the phenotypic MIC provided by culture-based methods integrates the net effect of all resistance mechanisms present in the bacterium, providing a direct, functional measure that correlates with clinical outcome. This provides the definitive data required to validate the predictions made by faster, genotypic or AI-driven platforms.
The Trajectory of Molecular and AI-Driven Methods
The pipeline for rapid AST is robust, with over 90 technologies identified in a recent scoping review [97]. The World Health Organization (WHO) emphasizes the critical need for diagnostics suitable for low-resource settings, including tools that bypass the need for culture [100]. AI and machine learning technologies are emerging as powerful auxiliary tools, capable of predicting pathogen antibiotic resistance by extracting in-depth information from microscopy images, MALDI-TOF spectra, or genomic data [98]. These technologies hold significant potential for infection monitoring and optimizing antibacterial drug use.
However, the ultimate validation of these innovative platforms still rests on their correlation with the phenotypic results provided by culture-based methods. As these new technologies evolve, the "enduring niche of culture" will likely shift from a primary testing method to the indispensable gold standard for calibrating and validating the next generation of rapid, point-of-care AST diagnostics. The future of AST lies not in the displacement of culture by molecular methods, but in a synergistic model where culture provides the validated foundation upon which faster, more accessible technologies are built and trusted.
Conclusion
The comparison between traditional culture and molecular methods reveals a clear trajectory in pathogen discovery toward faster, more sensitive, and culture-independent diagnostics. Techniques like mNGS and ddPCR are proving indispensable for rapid outbreak response and identifying elusive pathogens, significantly outperforming culture in detection rates and speed. However, culture retains its critical role in providing live isolates for antimicrobial susceptibility testing and public health surveillance. The future lies not in replacement, but in a synergistic diagnostic model. This integrated approach, combined with ongoing innovation to make molecular tools more accessible and affordable, promises to revolutionize our capacity to detect and respond to emerging microbial threats, ultimately enhancing patient outcomes and global health security.
References
- 1. Clinical microbiology in detection and identification of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9639501/]
- 2. Microbiological methodologies: Comparative evaluation of ... [https://www.sciencedirect.com/science/article/pii/S0717345825000053]
- 3. Are Bacterial Culture Quantifications Reliable? ... [https://pubmed.ncbi.nlm.nih.gov/30307844/]
- 4. Pathogen Discovery in the Post-COVID Era - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC10821006/]
- 5. Molecular techniques v. conventional culture: Should there ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12490501/]
- 6. Diagnostic comparison of microbial culture and ... [https://www.sciencedirect.com/science/article/pii/S277270762500178X]
- 7. Comparison of pathogen detection consistency between ... [https://www.nature.com/articles/s41598-023-36681-5]
- 8. MilliDrop AzurEvo [https://www.goldstandarddiagnostics.com/instruments/millidrop-azurevo.html]
- 9. Pathogen Discovery, Detection, and Diagnostics - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK221492/]
- 10. Cultivation strategies for growth of uncultivated bacteria [https://pmc.ncbi.nlm.nih.gov/articles/PMC5382963/]
- 11. Fastidious organism [https://en.wikipedia.org/wiki/Fastidious_organism]
- 12. Current Perspectives on Viable but Non-Culturable (VBNC) ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4116801/]
- 13. Viable But Non-Culturable (VBNC) Bacteria: Threats and ... [https://krakensense.com/blog/viable-but-non-culturable-vbnc-bacteria-threats-and-solutions]
- 14. Contemporary diagnostics for medically relevant fastidious ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9300619/]
- 15. Challenges of unculturable bacteria: environmental ... [https://link.springer.com/article/10.1007/s11157-020-09522-4]
- 16. Comparing culture and molecular methods for the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5100109/]
- 17. Comparison of Culture and Molecular Identification ... [https://www.mdpi.com/1422-0067/13/3/2535]
- 18. Diagnostic Innovations to Combat Antibiotic Resistance in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12428289/]
- 19. Determinants of empiric combination antibiotic therapy for ... [https://www.nature.com/articles/s41598-025-22687-8]
- 20. Recent Advances in the Use of Molecular Methods for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9229729/]
- 21. Molecular Methods for Pathogenic Bacteria Detection and ... [https://www.mdpi.com/2073-4441/13/24/3551]
- 22. Antibiotics, Volume 14, Issue 8 (August 2025) – 115 articles [https://www.mdpi.com/2079-6382/14/8]
- 23. Rapid clinical diagnosis and treatment of common ... [https://www.medrxiv.org/content/10.1101/2025.01.08.25320182v2]
- 24. Artificial intelligence in bacterial diagnostics and ... [https://www.sciencedirect.com/science/article/abs/pii/S0956566325002738]
- 25. Deep-learning-enabled antibiotic discovery through ... [https://www.nature.com/articles/s41551-024-01201-x]
- 26. Perspective The Science of Antibiotic Discovery [https://www.sciencedirect.com/science/article/pii/S0092867420302336]
- 27. Strategies for Natural Products Discovery from Uncultured ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8156983/]
- 28. New insights from uncultivated genomes of the global ... [https://www.nature.com/articles/s41586-019-1058-x]
- 29. Culturing the uncultured anaerobes from the perspective of ... [https://www.sciencedirect.com/science/article/abs/pii/S0966842X25001829]
- 30. A 'Culture' Shift: Application of Molecular Techniques for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6447436/]
- 31. Comparative analysis of three experimental methods for ... [https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-025-03985-7]
- 32. Molecular and Culture-Based Assessment of the Microbial ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3405613/]
- 33. Comparative Use of Quantitative PCR (qPCR), Droplet ... [https://www.mdpi.com/2073-4441/12/12/3507]
- 34. Absolute vs. Relative Quantification for qPCR [https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-learning-center/real-time-pcr-basics/absolute-vs-relative-quantification-real-time-pcr.html]
- 35. Droplet digital PCR vs. quantitative real time ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10440704/]
- 36. Comparison of digital PCR methods [https://www.qiagen.com/us/knowledge-and-support/knowledge-hub/bench-guide/pcr/digital-pcr/comparison-of-digital-pcr-methods]
- 37. Comparative Performance of Digital PCR and Real-Time RT ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12474457/]
- 38. Relative versus absolute RNA quantification [https://pmc.ncbi.nlm.nih.gov/articles/PMC7883443/]
- 39. dPCR vs. ddPCR: Why Precision Matters in Cell and Gene ... [https://www.roslinct.com/insights/dpcr-vs-ddpcr/]
- 40. Clinical applications of metagenomics next-generation ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11199093/]
- 41. A retrospective analysis comparing metagenomic next ... [https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2025.1530486/full]
- 42. Experience of implementing metagenomic next-generation ... [https://www.nature.com/articles/s41598-025-94840-2]
- 43. Metagenomic next-generation sequencing versus ... [https://www.nature.com/articles/s41598-023-31974-1]
- 44. Seven-year performance of a clinical metagenomic next- ... [https://www.nature.com/articles/s41591-024-03275-1]
- 45. Metagenomic next-generation sequencing (mNGS) versus tissue culture technique (TCT) in diagnosis of spinal infection: a systematic review and meta-analysis | Scientific Reports [https://www.nature.com/articles/s41598-025-06759-3]
- 46. Frontiers | Comparison and evaluation of metagenomic next-generation sequencing (mNGS) and real-time PCR for the detection of Mycobacterium tuberculosis [https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2025.1694179/full]
- 47. Metagenomic Next-Generation Sequencing vs. Traditional ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8814106/]
- 48. Diagnostic performance and clinical utility of metagenomic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12375686/]
- 49. CRISPR revolution: Unleashing precision pathogen ... [https://www.sciencedirect.com/science/article/pii/S104620232500115X]
- 50. CRISPRâ€driven diagnostics: Molecular mechanisms, clinical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12464570/]
- 51. Review The CRISPR-Cas system in molecular diagnostics [https://www.sciencedirect.com/science/article/abs/pii/S0009898124020722]
- 52. CRISPR-Based Diagnostics Market Size to Hit USD 15.14 ... [https://www.precedenceresearch.com/crispr-based-diagnostics-market]
- 53. Integrating AI and CRISPR Cas13a for rapid detection of ... [https://www.nature.com/articles/s41598-025-11405-z]
- 54. Viral Infections of the Central Nervous System: A Case-Based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2801692/]
- 55. A Comparison of Blood Pathogen Detection Among ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2021.641202/full]
- 56. A comparative analysis of sepsis identification methods in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5851804/]
- 57. Respiratory Virus Activity Levels [https://www.cdc.gov/respiratory-viruses/data/activity-levels.html]
- 58. Respiratory Illnesses Data Channel [https://www.cdc.gov/respiratory-viruses/data/index.html]
- 59. Influenza, SARS-CoV-2, RSV and other respiratory viruses [https://www.paho.org/en/topics/influenza-sars-cov-2-rsv-and-other-respiratory-viruses]
- 60. Is it time to substitute culture with molecular diagnostics for ... [https://www.thermofisher.com/blog/clinical-conversations/is-it-time-to-substitute-culture-with-molecular-diagnostics-for-infectious-diseases/]
- 61. Pathogen Testing: Immunoassay vs. Molecular Methods [https://www.eurofinsus.com/food-testing/resources/pathogen-testing-immunoassay-vs-molecular-methods/]
- 62. Detecting pathogens faster and more accurately by melting ... [https://www.amp.org/about/newsroom/amp-blog-content/detecting-pathogens-faster-and-more-accurately-by-melting-dna/]
- 63. A Comparative Study of Broad-Range Real-Time PCR and ... [https://www.mdpi.com/2075-4418/15/8/966]
- 64. Comparison of microbial culture, metagenomic next- ... [https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2025.1606283/full]
- 65. Comparative analysis of three experimental methods for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12039119/]
- 66. Traditional and Molecular Techniques for the Study of ... [https://wwwnc.cdc.gov/eid/article/8/2/01-0141_article]
- 67. 5 Emerging Wastewater Treatment Technologies to Watch ... [https://www.pmap.ca/post/wastewater-treatment-emerging-technologies]
- 68. Hidden Breakthroughs in Wastewater Treatment ... [https://susbio.in/wastewater-treatment-technologies-2025/]
- 69. Technology status to treat PFAS-contaminated water and ... [https://www.nature.com/articles/s41545-025-00457-3]
- 70. Comparison of traditional culture and molecular qPCR for ... [https://www.nature.com/articles/s41598-017-04915-y]
- 71. Comparative diagnostic performance of metagenomic and ... [https://www.nature.com/articles/s41598-025-11834-w]
- 72. Science unlocked: publication picks from October 2025 [https://nanoporetech.com/blog/science-unlocked-publication-picks-from-october-2025]
- 73. Clinical Microbiology in the Year 2025 - PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC139721/]
- 74. From Tradition to Innovation: Diverse Molecular ... [https://www.mdpi.com/2075-4418/14/24/2876]
- 75. reducing turnaround time and improving clinical outcomes [https://pubmed.ncbi.nlm.nih.gov/41217182/]
- 76. Comparison evaluation of bacterial DNA extraction ... [https://www.nature.com/articles/s41598-025-87225-y]
- 77. Organizing and Optimizing Laboratory Space for Molecular ... [https://www.aphl.org/programs/newborn_screening/Pages/Molecular-Workflow.aspx]
- 78. Cell Culture Workflow Solutions [https://www.thermofisher.com/us/en/home/life-science/cell-culture/mammalian-cell-culture/cell-culture-workflow-solutions.html]
- 79. Rapid, season-specific PCR testing versus traditional ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12318422/]
- 80. Comparison of microbial culture, metagenomic next ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12343261/]
- 81. Fast but accurate: a systematic review and meta-analysis ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2025.1703247/full]
- 82. Utilization of a Multi-modal Comprehensive Genomic and ... [https://link.springer.com/article/10.1007/s40291-025-00793-7]
- 83. Pathogen detection by multiplex PCR: A comparative study ... [https://www.sciencedirect.com/science/article/pii/S2212534525001212]
- 84. Molecular Testing: A Game-Changer for GI Infections [https://biosafesolutions.com/molecular-testing-gastrointestinal-diagnostics/]
- 85. Laboratory Turnaround Time - PMC - PubMed Central - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC2282400/]
- 86. Enhancing the Time-to-Positivity Paradigm for Guidance of ... [https://www.sciencedirect.com/science/article/abs/pii/S0098135425003278]
- 87. Time to positivity as a predictor of catheter-related bacteremia ... [https://ccforum.biomedcentral.com/articles/10.1186/s13054-025-05292-z]
- 88. Determinants of time to positivity in bloodstream infections [https://link.springer.com/article/10.1007/s10096-025-05096-7]
- 89. Time to positivity for differentiating blood culture ... [https://pubmed.ncbi.nlm.nih.gov/40768822/]
- 90. Comparison of pathogen detection performance between ... [https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2025.1563962/full]
- 91. Polymicrobial Infections: A Comprehensive Review on ... [https://www.mdpi.com/2813-9054/70/4/39]
- 92. Rapid clinical diagnosis and treatment of common, ... [https://www.medrxiv.org/content/10.1101/2025.01.08.25320182v1.full-text]
- 93. Current and Future Technologies for the Detection of Antibiotic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10606640/]
- 94. Detecting antibiotic resistance: classical, molecular ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2025.1673343/full]
- 95. Comparison of pathogen detection performance between ... [https://pubmed.ncbi.nlm.nih.gov/40861485/]
- 96. Antimicrobial Susceptibility Testing: A Comprehensive Review ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9024665/]
- 97. Next-generation rapid phenotypic antimicrobial ... [https://www.nature.com/articles/s41467-024-53930-x]
- 98. Advancements in AI-driven drug sensitivity testing research [https://pmc.ncbi.nlm.nih.gov/articles/PMC12081381/]
- 99. Updating Breakpoints in Antimicrobial Susceptibility Testing [https://asm.org/articles/2022/february/updating-breakpoints-in-antimicrobial-susceptibili]
- 100. WHO releases new reports on new tests and treatments in ... [https://www.who.int/news/item/02-10-2025-who-releases-new-reports-on-new-tests-and-treatments-in-development-for-bacterial-infections]